Worked Example Determining Solute Concentration By Acid Base Titration Khan Academy

Worked Example Determining Solute Concentration By Acidвђ Bas Courses on khan academy are always 100% free. start practicing—and saving your progress—now! khanacademy.org science ap chemistry beta x2eef969c7. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). in an acid base titration, the titrant is a strong base or a strong acid, and the analyte is an acid or a base, respectively. the point in a titration when the titrant and analyte are present in stoichiometric amounts is called the equivalence point. this point.

Solution Acid Base Titration Curves Titrations Chemical Processes Mcat Learn how to calculate the concentration of an unknown solute using acid–base titration. watch a worked example of a titration problem and practice with exercises. If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. In a strong acid strong base titration, the acid and base will react to form a neutral solution. at the equivalence point of the reaction, hydronium (h ) and hydroxide (oh ) ions will react to form water, leading to a ph of 7. this is true of all strong acid strong base titrations. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). in an acid base titratio.

How To Compute Molarity Determining Solute Concentration By Acid Base In a strong acid strong base titration, the acid and base will react to form a neutral solution. at the equivalence point of the reaction, hydronium (h ) and hydroxide (oh ) ions will react to form water, leading to a ph of 7. this is true of all strong acid strong base titrations. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). in an acid base titratio. Keep going! check out the next lesson and practice what you’re learning: khanacademy.org science ap chemistry beta x2eef969c74e0d802:acids and bas. Acid–base titrimetry is a standard method for the quantitative analysis of many inorganic acids and bases. a standard solution of naoh can be used to determine the concentration of inorganic acids, such as h 3 po 4 or h 3 aso 4, and inorganic bases, such as na 2 co 3 can be analyzed using a standard solution of hcl.
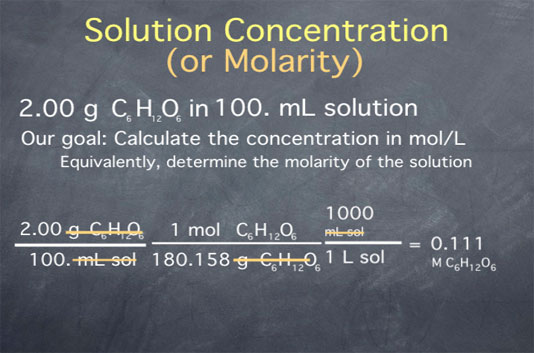
How To Compute Molarity Determining Solute Concentration By Acid Base Keep going! check out the next lesson and practice what you’re learning: khanacademy.org science ap chemistry beta x2eef969c74e0d802:acids and bas. Acid–base titrimetry is a standard method for the quantitative analysis of many inorganic acids and bases. a standard solution of naoh can be used to determine the concentration of inorganic acids, such as h 3 po 4 or h 3 aso 4, and inorganic bases, such as na 2 co 3 can be analyzed using a standard solution of hcl.

Comments are closed.