Women Who Have Option Of Using Hpv Self Sampling Kits More Likely To

Women Who Have Option Of Using Hpv Self Sampling Kits More Likely To Self sampling kits were delivered using either opt out or opt in approaches. opt out strategies included direct mailing and door to door delivery of self sampling kits by health workers. opt in dissemination required women to request the kits, which could be either mailed to their homes or picked up at another location. However, since then, self sampling was more actively promoted and the manner of requesting a kit became more easy, resulting in a substantial increase on the proportion of screened women using self sampling (16% in 2020) . while this invitation strategy might not be the key to overcome the low screening rates among under screened women, these.
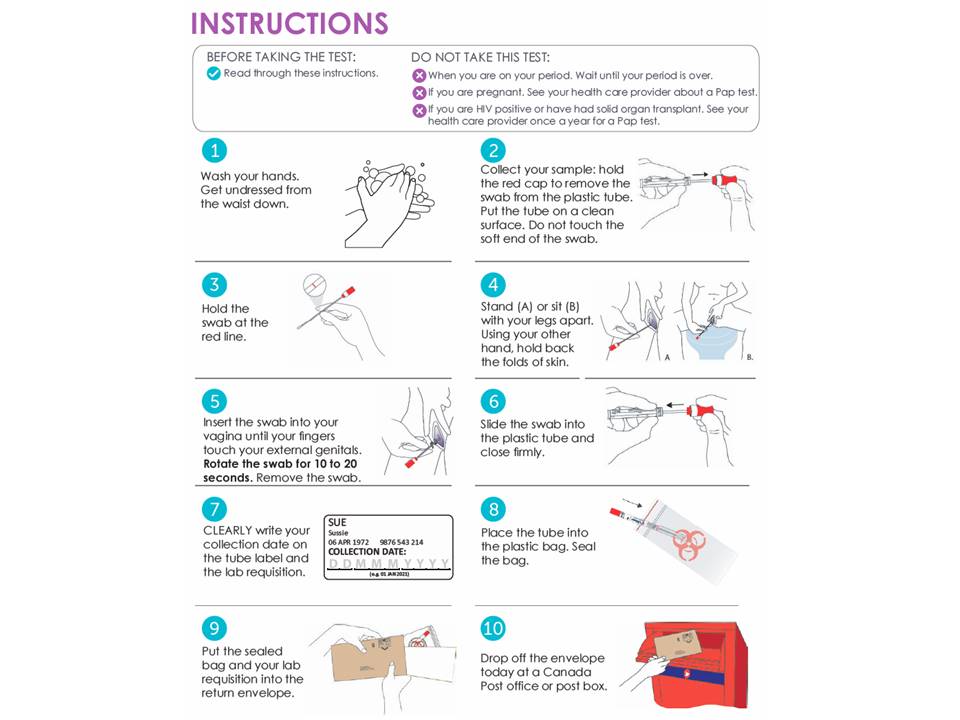
Using Hpv Tests For Cervical Cancer Screening And Managing Hpv Positive A recent study found that 72.7% of us women reported high willingness to use an hpv self sample kit at home 51 which is consistent with other studies from the us. 52–58 in the first randomized controlled trial evaluating the feasibility of mailing hpv self sampling kits to underscreened us women, more than half the women chose to return a. However, since then, self sampling was more actively promoted and the manner of requesting a kit became more easy, resulting in a substantial increase on the proportion of screened women using. Youscreen has now shown that hpv self sampling kits, which can be used privately, have the potential to bring the benefits of cervical screening to many more people. “ self sampling has been hailed as a game changer for cervical screening and we now have evidence in a uk population to show that it really is,” said dr anita lim, the trial. For the first time, women in the united states have the ability to self collect a sample to screen for human papillomavirus (hpv). this week, the u.s. food and drug administration approved two.

Hpv Self Collection Kit Cervical Swab вђ Medilabz Youscreen has now shown that hpv self sampling kits, which can be used privately, have the potential to bring the benefits of cervical screening to many more people. “ self sampling has been hailed as a game changer for cervical screening and we now have evidence in a uk population to show that it really is,” said dr anita lim, the trial. For the first time, women in the united states have the ability to self collect a sample to screen for human papillomavirus (hpv). this week, the u.s. food and drug administration approved two. A kit requested by 485 individuals. b 39 other high risk hpv positive only and 5 unsatisfactory results. c 32 other high risk hpv positive only and 7 unsatisfactory results. d 2 returned kits untested (1 received beyond specimen stability and 1 with mold on the swab) and rescreened in clinic <6 mo postrandomization; and 6 returned a tested kit after in clinic screening (kit results did not. For now, the option to self collect a vaginal sample for hpv testing must be done in a health care setting. on may 14, the food and drug administration (fda) expanded the approvals of two tests that detect cancer causing types of human papillomavirus (hpv) in the cervix. both tests are used as part of screening for cervical cancer.

Hpv Mailed Self Sampling Kits Help More Women Get Screened For T A kit requested by 485 individuals. b 39 other high risk hpv positive only and 5 unsatisfactory results. c 32 other high risk hpv positive only and 7 unsatisfactory results. d 2 returned kits untested (1 received beyond specimen stability and 1 with mold on the swab) and rescreened in clinic <6 mo postrandomization; and 6 returned a tested kit after in clinic screening (kit results did not. For now, the option to self collect a vaginal sample for hpv testing must be done in a health care setting. on may 14, the food and drug administration (fda) expanded the approvals of two tests that detect cancer causing types of human papillomavirus (hpv) in the cervix. both tests are used as part of screening for cervical cancer.
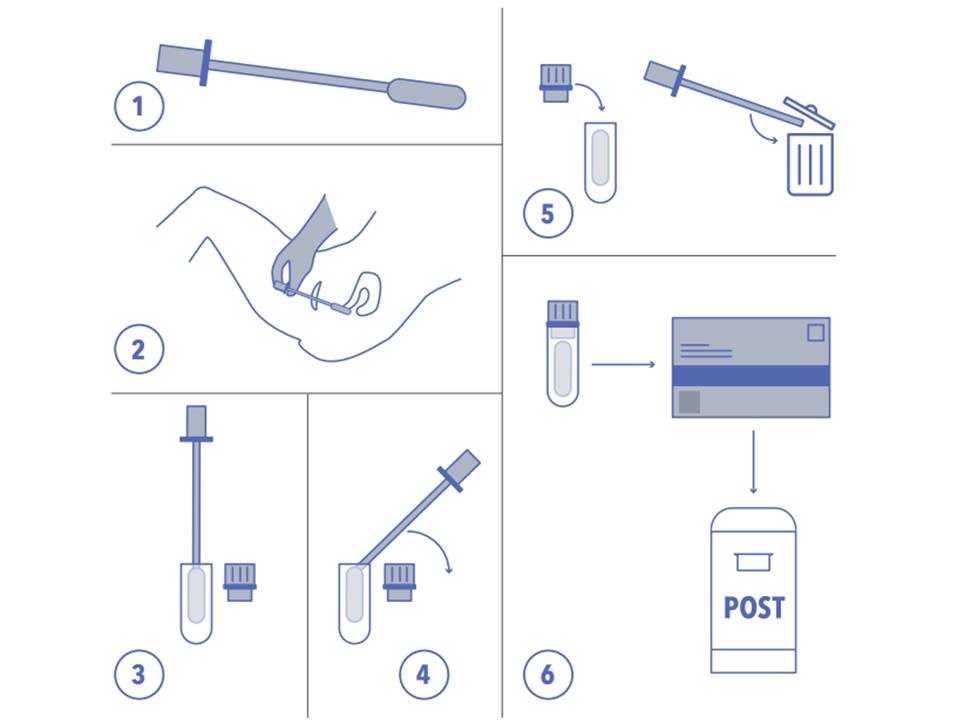
Using Hpv Tests For Cervical Cancer Screening And Managing Hpv Positive

Comments are closed.