Vsepr Theory W3schools
Vsepr Theory W3schools Vsepr theory. the model of valence shell electron pair replication is oftentimes abbreviated as vsepr (known as “vesper”). it is fundamentally a model which predicts a molecule’s geometry. in particular, vsepr models see the molecular geometry and bonding of polyatomic ions and organic molecules. it is beneficial for almost the whole of. Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (figure 10.2.4 10.2. 4). thus bonding pairs and lone pairs repel each other electrostatically in the order bp–bp < lp–bp < lp–lp. in so 2, we have one bp–bp interaction and two lp–bp interactions. 4.

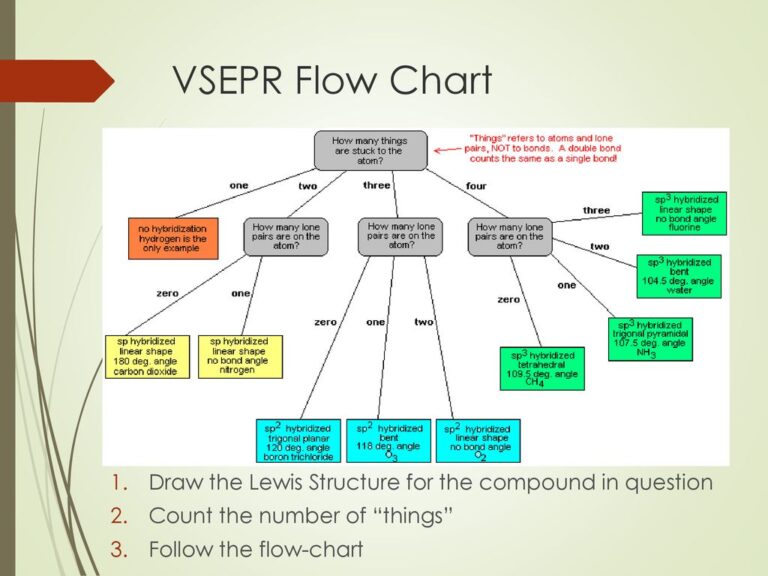
Vsepr Theory W3schools The bond angle for water is 104.5°. valence shell electron pair repulsion (vsepr) theory ( ˈvɛspər, vəˈsɛpər vesp ər, [1]: 410 və sep ər[2]) is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. [3] it is also named the gillespie nyholm. According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o. The valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced "vesper") and is a model to predict the geometry of molecules. specifically, vsepr models look at the bonding and molecular geometry of organic molecules and polyatomic ions. it is useful for nearly all compounds that have a central atom that is not a metal. lewis structures only tell the number. The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. the theory is based on the idea of minimizing the electrostatic repulsion between electron pairs, as first proposed by sidgwick and powell in 1940, [9] then generalized by gillespie and nyholm in 1957, [10] and.

Comments are closed.