Vsepr Theory Basic Introduction
Vsepr Theory Basic Introduction Channels For Pearson This chemistry video tutorial provides a basic introduction into vsepr theory and molecular structure. it contains examples and practice problems of drawing. Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (figure 10.2.4 10.2. 4). thus bonding pairs and lone pairs repel each other electrostatically in the order bp–bp < lp–bp < lp–lp. in so 2, we have one bp–bp interaction and two lp–bp interactions. 4.

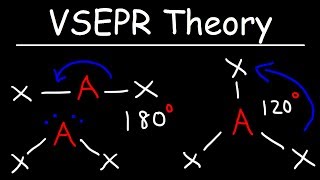
Cc A Painless Introduction To Vsepr Theory This chemistry video tutorial provides a basic introduction into molecular geometry and vsepr theory. examples and practice problems include the trigonomal. To see all my chemistry videos, check out socratic.org chemistrythis is an introduction to the basics of vsepr theory. vsepr theory is a set of rules f. The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. the theory is based on the idea of minimizing the electrostatic repulsion between electron pairs, as first proposed by sidgwick and powell in 1940, [9] then generalized by gillespie and nyholm in 1957, [10] and then broadly applied over the intervening 50 years. According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o.

Comments are closed.