Vsepr Theory And Shapes Of Molecules
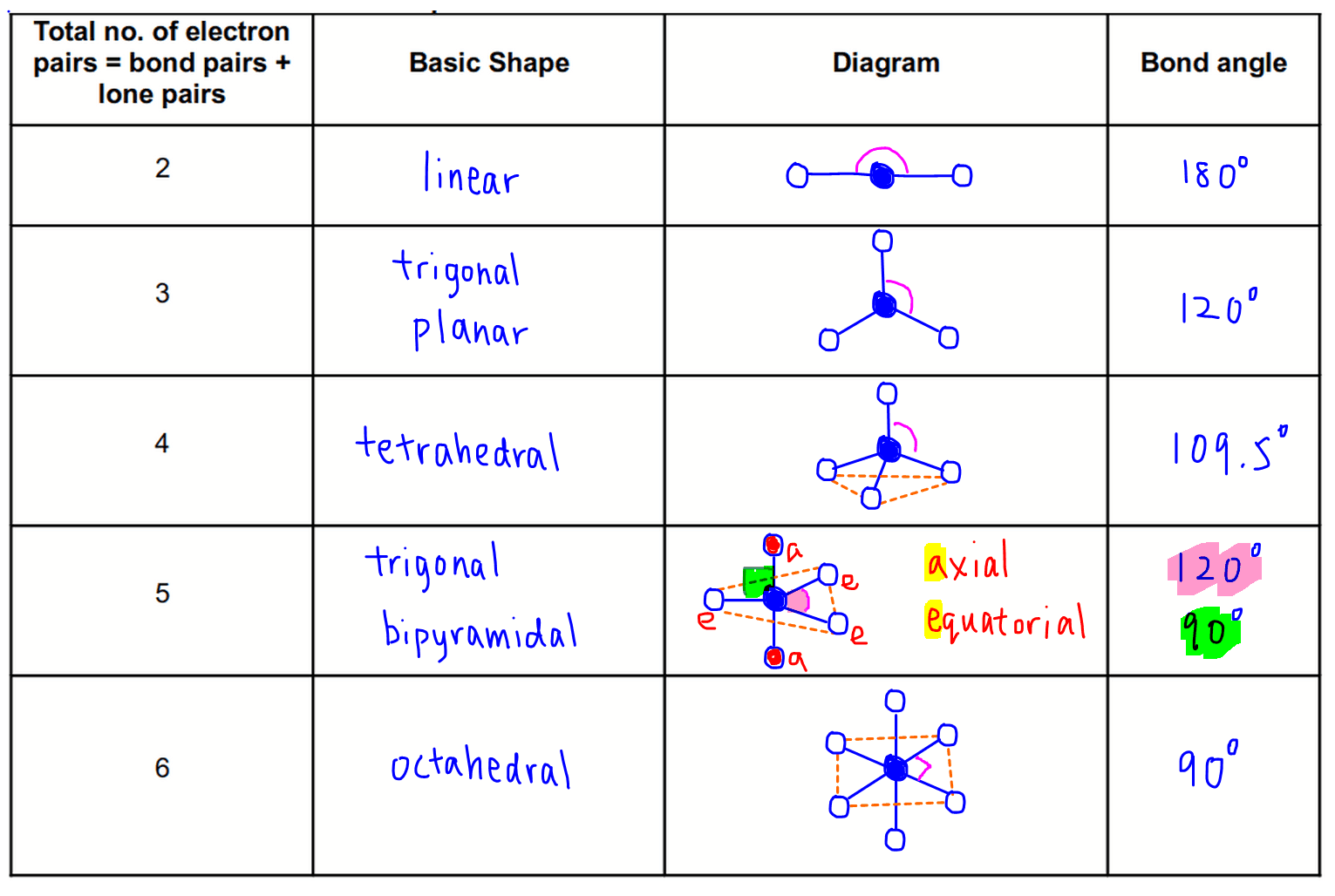
Vsepr Theory And Shapes Of Molecules The vsepr model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. a combination of vsepr and a bonding model, such as lewis electron structures, is necessary to understand the presence of multiple bonds. The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. the theory is based on the idea of minimizing the electrostatic repulsion between electron pairs, as first proposed by sidgwick and powell in 1940, [9] then generalized by gillespie and nyholm in 1957, [10] and.

Shape Of Molecules Vsepr Theory Affect Shape Of The Molecule The shapes of these molecules can be predicted from their lewis structures, however, with a model developed about 30 years ago, known as the valence shell electron pair repulsion (vsepr) theory. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of. When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. examples include beh 2 and co 2: figure 4.13.1 4.13. 1 beryllium hydride and carbon dioxide bonding. the two molecules, shown in the figure below in a "ball and stick" model. Introduction. this section explores how we predict the molecular and electron pair shapes of molecules using the vsepr (valence shell electron pair repulsion) theory. we will first go over what vsepr theory is and how it defines an electron pair geometry and a molecular geometry. then we will go over the steps for determining the electron pair. Stefanie sydlik explains how her research on designing sensors for explosives depends on the principles of vsepr (valence shell electron pair repulsion) theory. stefanie’s sensor design amplifies small scale changes in bond angles following interaction with a target molecule, and she hopes that her work will lead to better detectors for.

Vsepr Theory Chart Model Lesson Study Introduction. this section explores how we predict the molecular and electron pair shapes of molecules using the vsepr (valence shell electron pair repulsion) theory. we will first go over what vsepr theory is and how it defines an electron pair geometry and a molecular geometry. then we will go over the steps for determining the electron pair. Stefanie sydlik explains how her research on designing sensors for explosives depends on the principles of vsepr (valence shell electron pair repulsion) theory. stefanie’s sensor design amplifies small scale changes in bond angles following interaction with a target molecule, and she hopes that her work will lead to better detectors for. The bond angle for water is 104.5°. valence shell electron pair repulsion (vsepr) theory ( ˈvɛspər, vəˈsɛpər vesp ər, [1]: 410 və sep ər[2]) is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. [3] it is also named the gillespie nyholm. The shape ( ) of molecules influences physical and chemical properties, including melting point, boiling point, and reactivity. shape is particularly important in biological systems where, for example, a molecule must fit precisely into the active site of an enzyme. valence shell electron pair repulsion (vsepr) theory can be used to predict.

Chemical Bonding Molecular Shapes Vsepr Theory Britannica The bond angle for water is 104.5°. valence shell electron pair repulsion (vsepr) theory ( ˈvɛspər, vəˈsɛpər vesp ər, [1]: 410 və sep ər[2]) is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. [3] it is also named the gillespie nyholm. The shape ( ) of molecules influences physical and chemical properties, including melting point, boiling point, and reactivity. shape is particularly important in biological systems where, for example, a molecule must fit precisely into the active site of an enzyme. valence shell electron pair repulsion (vsepr) theory can be used to predict.
Vsepr Theory Psiberg

Comments are closed.