Valence Bond Theory Hybrid Orbitals And Molecular Orbital Theory
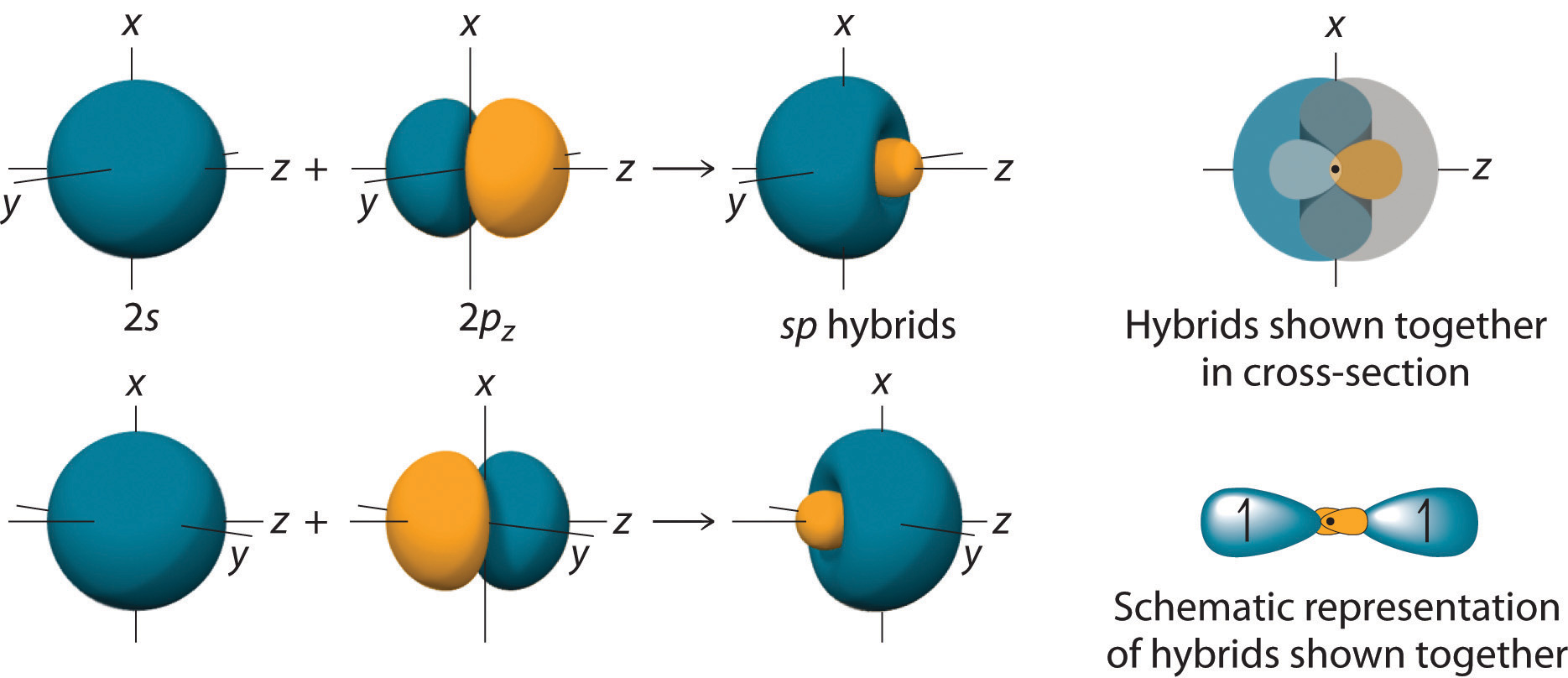
Chapter 5 2 Localized Bonding And Hybrid Orbitals Chemistry Libretexts Determine the geometry of the molecule using the strategy in example 10.7.1 10.7. 1. from the valence electron configuration of the central atom and the number of electron pairs, determine the hybridization. place the total number of electrons around the central atom in the hybrid orbitals and describe the bonding. The side by side overlap of two p orbitals gives rise to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, as shown in figure \(\pageindex{5}\). in valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron.
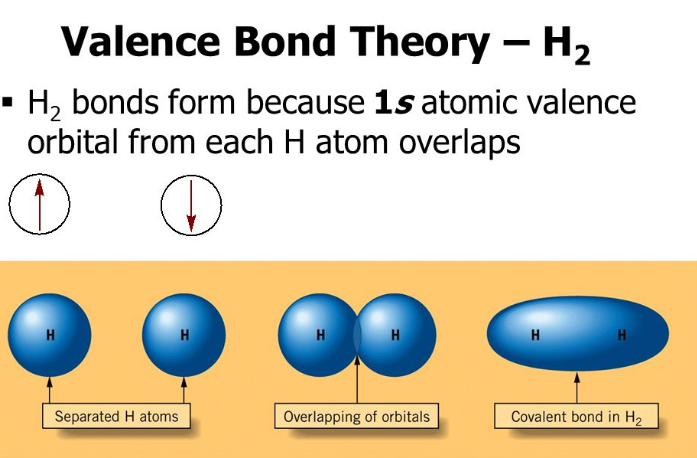
Valence Bond Vs Molecular Orbital Theory 10 Key Differences And The oxygen atomic orbitals are not oriented to allow the correct molecular geometry using valence bond theory. a different model is needed. figure 9.1.1 9.1. 1: the hypothetical overlap of two of the 2p orbitals on an oxygen atom (red) with the 1s orbitals of two hydrogen atoms (blue) would produce a bond angle of 90°. We said in section 1.5 that chemists use two models for describing covalent bonds: valence bond theory and molecular orbital theory. having now seen the valence bond approach, which uses hybrid atomic orbitals to account for geometry and assumes the overlap of atomic orbitals to account for electron sharing, let’s look briefly at the. Introductory chemistry textbooks often present valence bond (vb) theory as useful, but incorrect and inferior to molecular orbital (mo) theory, citing the electronic structure of o2 and electron delocalization as evidence. even texts that initially present the two theories on equal footing use language that biases students toward the mo approach. however, these “failures” of vb are really. As the names suggest, valence bond theory considers a molecule as a collection of bonds, whereas molecular orbital theory views the molecule as a whole. at the introductory level, neither theory is discussed anywhere close to its theoretical limit. vb is presented as a single con guration of electrons in fi hybrid orbitals, spin paired in bonds.

Comments are closed.