Understanding Chemical Kinetics

Chemical Kinetics Bartleby Chemical kinetics, the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. it is to be contrasted with thermodynamics, which deals with the direction in which a process occurs but in itself tells nothing about its rate. thermodynamics is time’s arrow, while chemical kinetics is time’s clock. Turnover number – number of catalyzed reactions occurring at a particular active site. large turnover numbers = low activation energies. 14.s: chemical kinetics (summary) is shared under a license and was authored, remixed, and or curated by libretexts. a summary of the key concepts in this chapter of the textmap created for "chemistry: the.

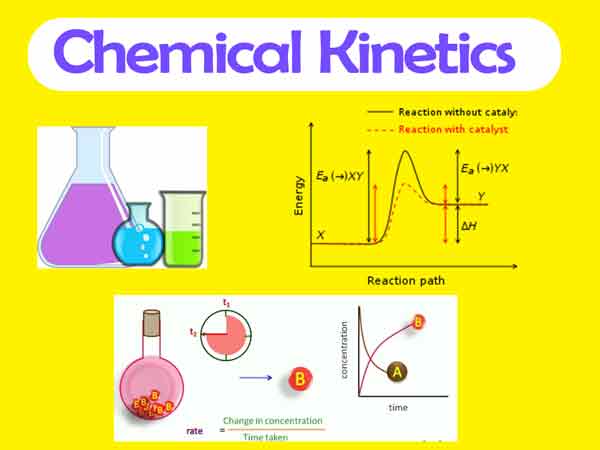
Chemical Kinetics Reaction Rates Equations And Mechanisms Geeksforgeeks Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. it is different from chemical thermodynamics , which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Key takeaways: chemical kinetics. chemical kinetics or reaction kinetic is the scientific study of the rates of chemical reactions.this includes the development of mathematical model to describe the rate of reaction and an analysis of the factors that affect reaction mechanisms. peter waage and cato guldberg are credited with pioneering the. 14.7: catalysis. catalysts participate in a chemical reaction and increase its rate. they do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The study of reaction rates, called chemical kinetics, encompasses a wide range of activities, measurements, and calculations. you might wonder why anyone would bother with this, but it turns out that we can use kinetic data to get more information about a reaction than just how fast it goes; we can find out about the pathway that the reaction.

Understanding Chemical Kinetics Chemicalkinetics Youtube 14.7: catalysis. catalysts participate in a chemical reaction and increase its rate. they do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The study of reaction rates, called chemical kinetics, encompasses a wide range of activities, measurements, and calculations. you might wonder why anyone would bother with this, but it turns out that we can use kinetic data to get more information about a reaction than just how fast it goes; we can find out about the pathway that the reaction. Chapter 4 chemical kinetics 2 not every collision leads to a reaction, but the rate of a reaction depends on the frequency with which reactant molecules collide, which is influenced by the concentration of the reactants (figure 1). the larger the number of reactant molecules that are confined within a given volume, the greater the. Why it matters: kinetics. figure 1. an agama lizard basks in the sun. as its body warms, the chemical reactions of its metabolism speed up. the lizard in figure 1 is not simply enjoying the sunshine or working on its tan. the heat from the sun’s rays is critical to the lizard’s survival. a warm lizard can move faster than a cold one because.
Chemical Kinetics Notes Chemical Kinetics Cbse Chemistry Topics Class 12 Chapter 4 chemical kinetics 2 not every collision leads to a reaction, but the rate of a reaction depends on the frequency with which reactant molecules collide, which is influenced by the concentration of the reactants (figure 1). the larger the number of reactant molecules that are confined within a given volume, the greater the. Why it matters: kinetics. figure 1. an agama lizard basks in the sun. as its body warms, the chemical reactions of its metabolism speed up. the lizard in figure 1 is not simply enjoying the sunshine or working on its tan. the heat from the sun’s rays is critical to the lizard’s survival. a warm lizard can move faster than a cold one because.

Comments are closed.