U3a5p1 Rates Of Reaction Pdf U3a5p1 Rates Of Reaction By Shivam

U3a5p1 Rates Of Reaction Pdf U3a5p1 Rates Of Reaction By Shivam View u3a5p1 rates of reaction.pdf from eng 336 at central connecticut state university. u3a5p1 rates of reaction by shivam gupta 1. a catalyst increases the speed of a reaction. The rate of reaction is defined as the change in concentration of a substance in unit time. its usual unit is mol dm 3s 1. when a graph of concentration of reactant is plotted vs time, the gradient of the curve is the rate of reaction. the initial rate is the rate at the start of the reaction where it is fastest.

Rate Of A Reaction Factors Affecting The Rate Of Reaction Chemistry Gradient = rate = Δ(product) Δ(time) it is quite common to want to know the initial rate of the reaction. this is found by drawing a tangent to the curve at time = 0 and finding the gradient at this point in the same way. from the shape of typical concentration vs. time graphs we can see that: the initial rate of reaction, at t=0, is the. Rates of chemical reactions are usually defined by comparing the change in reactant or product concentration over time. consider the reaction n2 3 h2 2 nh3. we could measure the rate at which n2 and h2 are consumed and the rate at which nh3 is produced. due to the stoichiometry of the reaction, the rate of n2 use will be 1 3 the rate of h2. 1. rate of reaction when the colliding molecules are dissimilar: consider a bimolecular reaction between different molecules a and b yielding product p as. → (137) the number of collisions between a and b occurring in the container per unit volume per unit time can be given by the following relation. 8 (138) = 2√. By "flooding" the reaction mixture with one or more reactants, we are effectively isolating the one in which we are interested. 17.1: rates of reactions and rate laws. chemical change is guided and driven by energetics, but the actual route it takes and the speed with which it occurs is the subject of "dynamics".

Lesson Video Rates Of Reactions Nagwa 1. rate of reaction when the colliding molecules are dissimilar: consider a bimolecular reaction between different molecules a and b yielding product p as. → (137) the number of collisions between a and b occurring in the container per unit volume per unit time can be given by the following relation. 8 (138) = 2√. By "flooding" the reaction mixture with one or more reactants, we are effectively isolating the one in which we are interested. 17.1: rates of reactions and rate laws. chemical change is guided and driven by energetics, but the actual route it takes and the speed with which it occurs is the subject of "dynamics". Equation 14 1.9 is a generic equation that can be used to relate the rates of production and consumption of the various species in a chemical reaction where capital letter denote chemical species, and small letters denote their stoichiometric coefficients when the equation is balanced. aa bb → cc dd. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction.
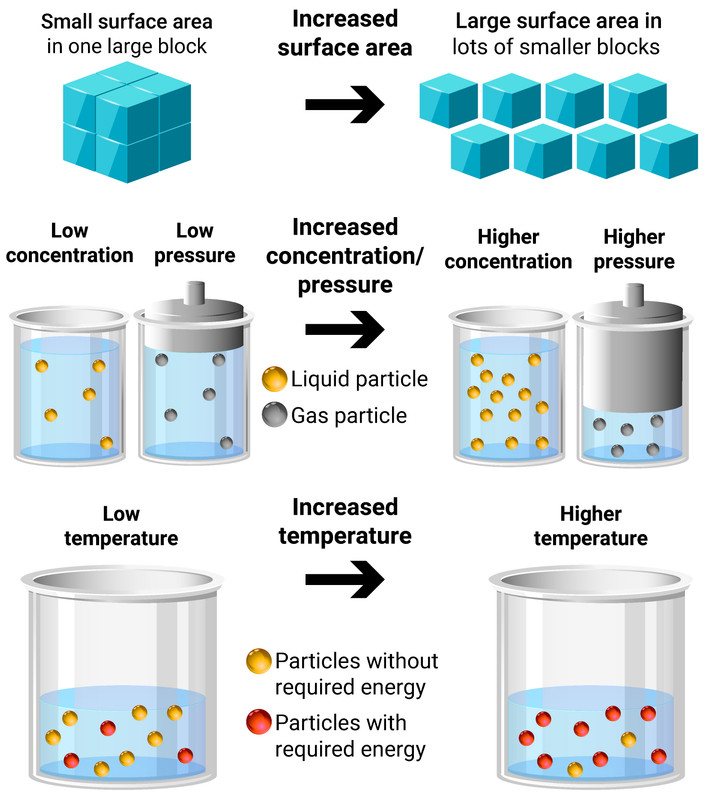
Energy Ws 1 Reaction Rates Equation 14 1.9 is a generic equation that can be used to relate the rates of production and consumption of the various species in a chemical reaction where capital letter denote chemical species, and small letters denote their stoichiometric coefficients when the equation is balanced. aa bb → cc dd. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction.

Comments are closed.