Types Of Matter Elements Compounds Mixtures And Pure Substances

6th Grade Science 1st Six Weeks Week 5 Pure Substances And Mixtures Matter can be broken down into two categories: pure substances and mixtures. pure substances are further broken down into elements and compounds. mixtures are physically combined structures that can be separated into their original components. a chemical substance is composed of one type of atom or molecule. a mixture is composed of different. It is a pure substance, and more specifically it is a compound with the formula of o 2. other examples of pure substances would be hydrogen, water, and table salt (sodium chloride, nacl) because they are all made up of only one component and their composition does not change from one sample to another. the mixtures, on the other hand, can be.
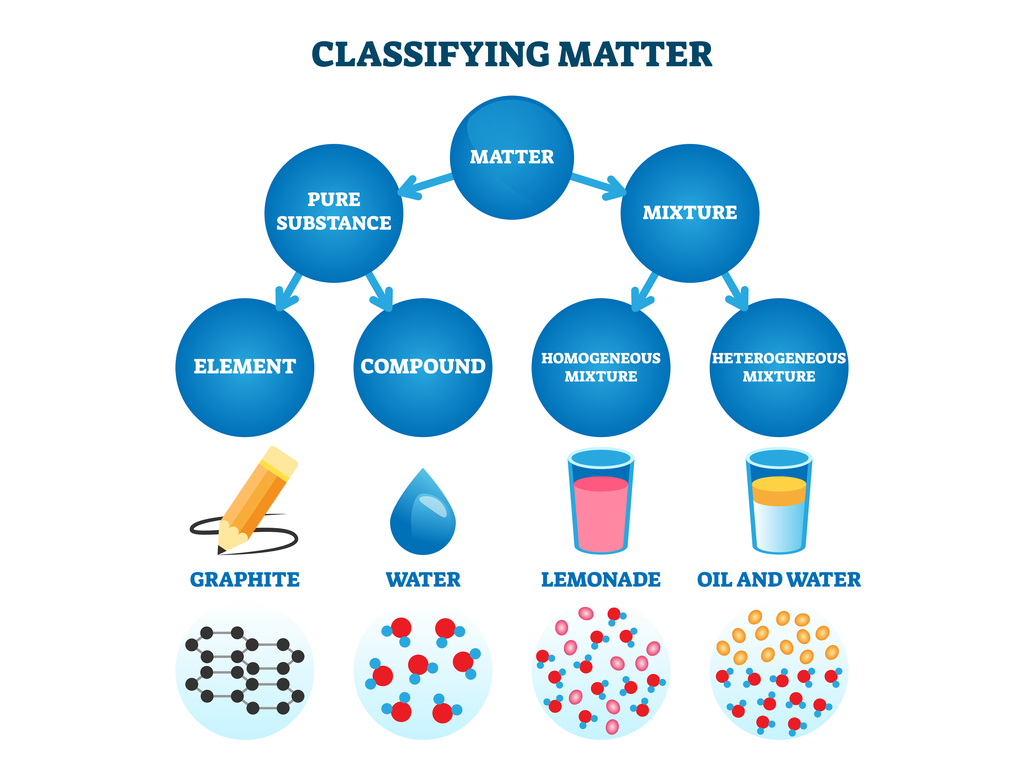
Differentiating Pure Substances And Mixtures вђ Lesson Science State This chemistry video tutorial provides a basic introduction into the different types of matter such as elements, compounds, mixtures, and pure substances.che. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. mixtures are physical combinations of two or more elements and or compounds. mixtures can be classified as homogeneous or heterogeneous. elements and compounds are both examples of pure substances. Figure 3.4.1 3.4. 1: relationships between the types of matter and the methods used to separate mixtures. ordinary table salt is called sodium chloride. it is considered a substance because it has a uniform and definite composition. all samples of sodium chloride are chemically identical. water is also a pure substance. Matter can be classified into two broad categories: pure substances and mixtures. a pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. mixtures are physical combinations of two or more elements and or compounds. mixtures can be classified as homogeneous or heterogeneous.

Pure Substance Vs Mixture Figure 3.4.1 3.4. 1: relationships between the types of matter and the methods used to separate mixtures. ordinary table salt is called sodium chloride. it is considered a substance because it has a uniform and definite composition. all samples of sodium chloride are chemically identical. water is also a pure substance. Matter can be classified into two broad categories: pure substances and mixtures. a pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. mixtures are physical combinations of two or more elements and or compounds. mixtures can be classified as homogeneous or heterogeneous. Pure substances that are comprised of two or more elements are called compounds. compounds may be broken down by chemical changes to yield either elements or other compounds, or both. for example, mercury (ii) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (figure 4). Matter can be classified into several categories. two broad categories are mixtures and pure substances. a pure substance has a constant composition. all specimens of a pure substance have exactly the same makeup and properties. any sample of sucrose (table sugar) consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass.

Comments are closed.