Types Of Chemical Reactions Synthesis Reactions Decomposition

Decomposition Reaction Definition Examples Applications 4 main types of chemical reactions. keep in mind, there are different names for the reaction types. the four main types of chemical reactions are: synthesis or combination reactions. decomposition or analysis reactions. single replacement, single displacement, or substitution reactions. double replacement, double displacement, or metathesis. A reaction can be classified as physical, chemical or nuclear based on the changes from reactants to products. common types of chemical reactions are synthesis, decomposition, single displacement, double displacement, combustion (burning of methane) and acid base (antacid with hcl) reactions.
Explain How Synthesis Reaction Differs From A Decomposition Reaction A typical example of a synthesis reaction is the formation of table salt. sodium and chlorine ions interact to form sodium chloride. decomposition reaction. a decomposition reaction occurs when the reactant breaks down into simpler products. here is the general equation that represents this type of reaction: unlike synthesis reactions. The five basic types of chemical reactions are combination, decomposition, single replacement, double replacement, and combustion. analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. some reactions will fit into more than one category. Synthesis reactions. a synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. the reactants may be elements or compounds, while the product is always a compound. the general equation for a synthesis reaction is: a b → ab. Most chemical reactions can be classified into one or more of five basic types: acid–base reactions, exchange reactions, condensation reactions (and the reverse, cleavage reactions), and oxidation–reduction reactions. the general forms of these five kinds of reactions are summarized in table 7.10.1, along with examples of each.

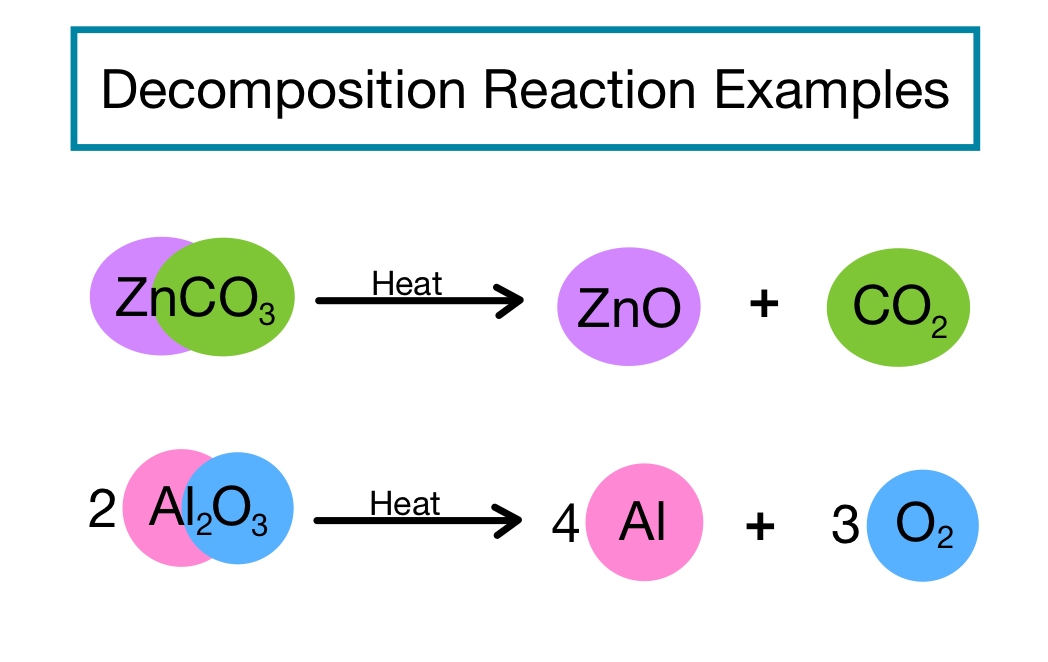
Explain How Synthesis Reaction Differs From A Decomposition Reaction Synthesis reactions. a synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. the reactants may be elements or compounds, while the product is always a compound. the general equation for a synthesis reaction is: a b → ab. Most chemical reactions can be classified into one or more of five basic types: acid–base reactions, exchange reactions, condensation reactions (and the reverse, cleavage reactions), and oxidation–reduction reactions. the general forms of these five kinds of reactions are summarized in table 7.10.1, along with examples of each. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. this type of reaction is also called a direct combination reaction or simply a combination reaction. it’s the type of reaction that forms compounds from their elements. synthesis reactions also make large. A decomposition reaction is the opposite of a synthesis reaction. in decomposition reactions, a single complex reactant breaks down into simpler products like elements, elements and compounds, or just compounds. decomposition reactions require an input of some form of energy.

Comments are closed.