Transition Metals In Ionic Formulas
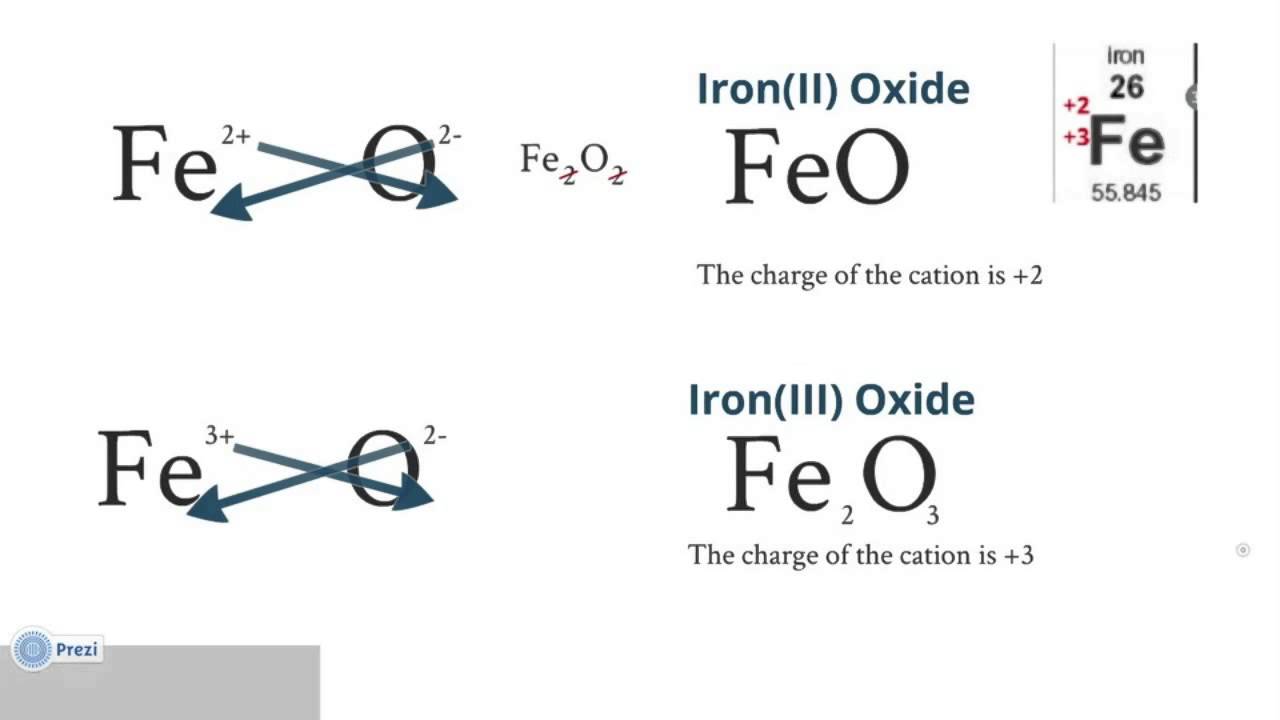
Ionic Compounds And Bonding Part 06 Transition Metal Nomenclature Transition metal ions. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. as an example, iron commonly forms two different ions. it can sometimes lose two electrons to form the fe2 fe 2 ion, while at other times it loses three electrons. The charges that can result upon the ionization of transition metals, as well as their corresponding roman numerals, are shown below in table 3.8.1 3.8. 1. therefore, using the iupac system, fe 2 is called the "iron (ii) ion," and fe 3 is named as the "iron (iii) ion." incorporating a roman numeral into the name of a transition metal cation.

Transition Metals In Ionic Formulas Youtube Watch on . naming ionic compounds with transition metals. write the name of transition metal as shown on the periodic table. write the name and charge for the non metal. if you have a polyatomic ion, use the common ion table to find and write the formula and charge. use the total charge on the non metal (or polyatomic ion) find the. The transition metals are unique because they are elements are able to we'll learn how to write formulas for ionic compounds that contain transition metals. The polarity of bonds with transition metals varies based not only upon the electronegativities of the atoms involved but also upon the oxidation state of the transition metal. remember that bond polarity is a continuous spectrum with electrons being shared evenly (covalent bonds) at one extreme and electrons being transferred completely (ionic. This chemistry video tutorial focuses on naming ionic compounds with polyatomic ions, transition metals & roman numerals. chemistry basic introduction:.

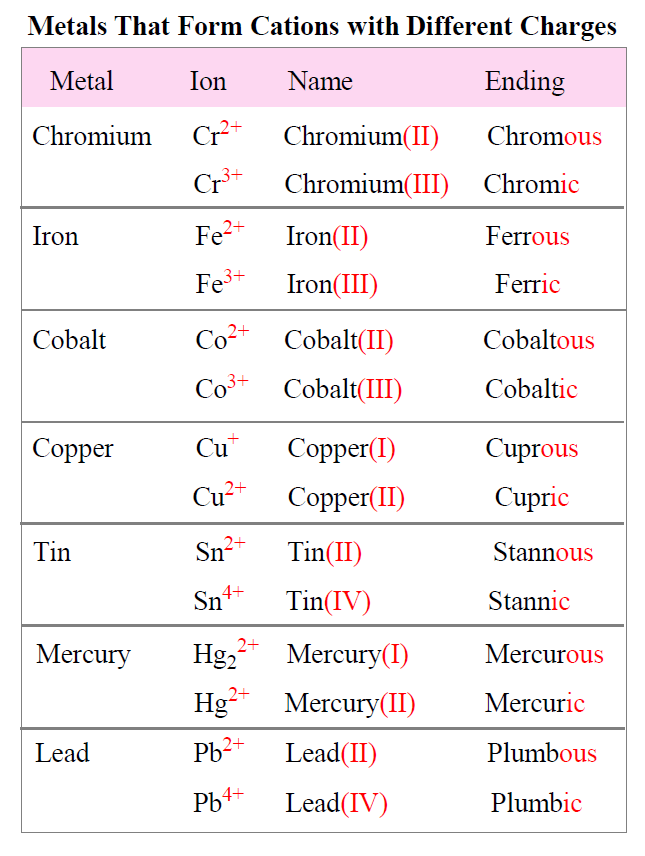
Formulas And Nomenclature Binary Ionic Transition Metals Wor The polarity of bonds with transition metals varies based not only upon the electronegativities of the atoms involved but also upon the oxidation state of the transition metal. remember that bond polarity is a continuous spectrum with electrons being shared evenly (covalent bonds) at one extreme and electrons being transferred completely (ionic. This chemistry video tutorial focuses on naming ionic compounds with polyatomic ions, transition metals & roman numerals. chemistry basic introduction:. Table 3.5.3 3.5. 3: names of some transition metal ionic compounds. out of date nomenclature used the suffixes – ic and – ous to designate metals with higher and lower charges, respectively: iron (iii) chloride, fecl 3, was previously called ferric chloride, and iron (ii) chloride, fecl 2, was known as ferrous chloride. Writing ionic formulas with transition metals. 1. intro to general chemistry 3h 51m. classification of matter. 16m. physical & chemical changes. 19m. chemical properties. 8m.
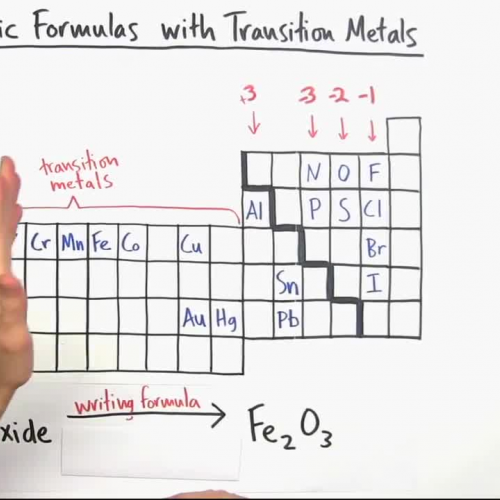
Writing Ionic Formulas With Transition Metals Table 3.5.3 3.5. 3: names of some transition metal ionic compounds. out of date nomenclature used the suffixes – ic and – ous to designate metals with higher and lower charges, respectively: iron (iii) chloride, fecl 3, was previously called ferric chloride, and iron (ii) chloride, fecl 2, was known as ferrous chloride. Writing ionic formulas with transition metals. 1. intro to general chemistry 3h 51m. classification of matter. 16m. physical & chemical changes. 19m. chemical properties. 8m.
Writing Chemical Formulas For Ionic Compounds Chemistry Steps

Comments are closed.