Thin Layer Chromatography Tlc Chemical Processes Mcat Khan

Thin Layer Chromatography Tlc Chemical Processes Mcat Khan Learn about how chemicals can be separated based on polarity through thin layer chromatography (tlc). by angela guerrero. . created by angela guerrero.watch. Course: mcat > unit 10. lesson 7: separations and purifications. separations and purifications questions. simple and fractional distillations. extractions. principles of chromatography. a closer look at types of chromatography. basics of chromatography. thin layer chromatography (tlc).

Thin Layer Chromatography Tlc Principle And Procedure Owlcation Thin layer chromatography (tlc) is a type of chromatography, where the stationary phase is a glass plate coated in the absorbent material (often silica gel or alumina) and the mobile phase is an organic solvent. in this case, non polar compounds are more soluble (higher rf values) and polar compounds are more adsorbent (lower rf values). Understand the basic principles of different kinds of chromatography: paper, thin layer, column, size exchange, ion exchange, affinity, hplc, and. by angela. Thin layer chromatography summary. place a small portion of solvent (5 5 10ml 10 ml for this chamber) into a tlc chamber with lid, along with a cut piece of filter paper. dissolve liquid or solid samples (1 drop per ∼ 1ml ∼ 1 ml solvent) using a low boiling solvent (e.g. acetone or dichloromethane). draw a pencil line on a tlc plate ∼. Khan academy.
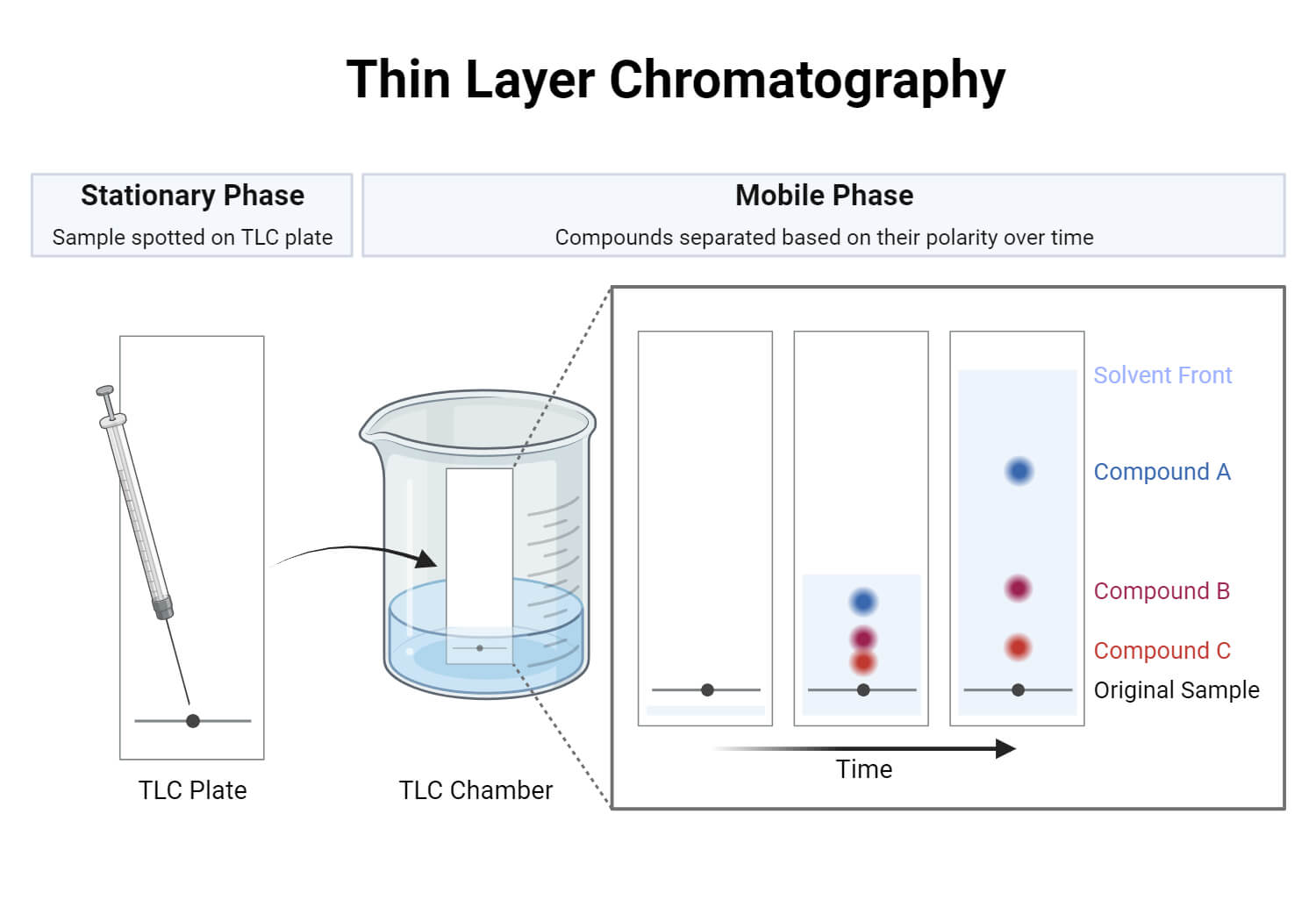
Thin Layer Chromatography Principle Parts Steps Uses Thin layer chromatography summary. place a small portion of solvent (5 5 10ml 10 ml for this chamber) into a tlc chamber with lid, along with a cut piece of filter paper. dissolve liquid or solid samples (1 drop per ∼ 1ml ∼ 1 ml solvent) using a low boiling solvent (e.g. acetone or dichloromethane). draw a pencil line on a tlc plate ∼. Khan academy. Procedure of thin layer chromatography (tlc) the stationary phase is applied onto the plate uniformly and then allowed to dry and stabilize. these days, however, ready made plates are more commonly used. with a pencil, a thin mark is made at the bottom of the plate to apply the sample spots. then, samples solutions are applied on the spots. Explanation: thin layer chromatography (tlc) is used to separate components in a mixture. components are separated on a tlc plate because each component travels a different distance. the distance travelled depends on several factors. one of those factors is polarity; therefore, tlc can used to determine polarity of substances.

Thin Layer Chromatography Tlc Intermolecular Forces And Properties Procedure of thin layer chromatography (tlc) the stationary phase is applied onto the plate uniformly and then allowed to dry and stabilize. these days, however, ready made plates are more commonly used. with a pencil, a thin mark is made at the bottom of the plate to apply the sample spots. then, samples solutions are applied on the spots. Explanation: thin layer chromatography (tlc) is used to separate components in a mixture. components are separated on a tlc plate because each component travels a different distance. the distance travelled depends on several factors. one of those factors is polarity; therefore, tlc can used to determine polarity of substances.

Comments are closed.