Thermodynamics A Relationships Between Heat And Other Forms Of Energy
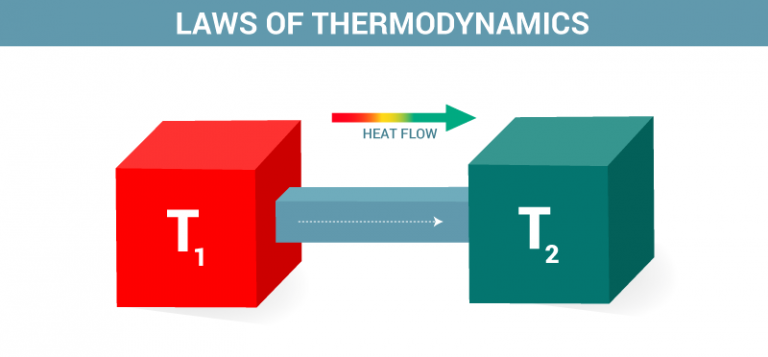
Thermodynamics A Relationships Between Heat And Other Forms Of Energy Thermodynamics, science of the relationship between heat, work, temperature, and energy. thermodynamics deals with the transfer of energy from one place to another and from one form to another. the key concept is that heat is a form of energy corresponding to a definite amount of mechanical work. Thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy. in particular, it describes how thermal energy is converted to and from other.
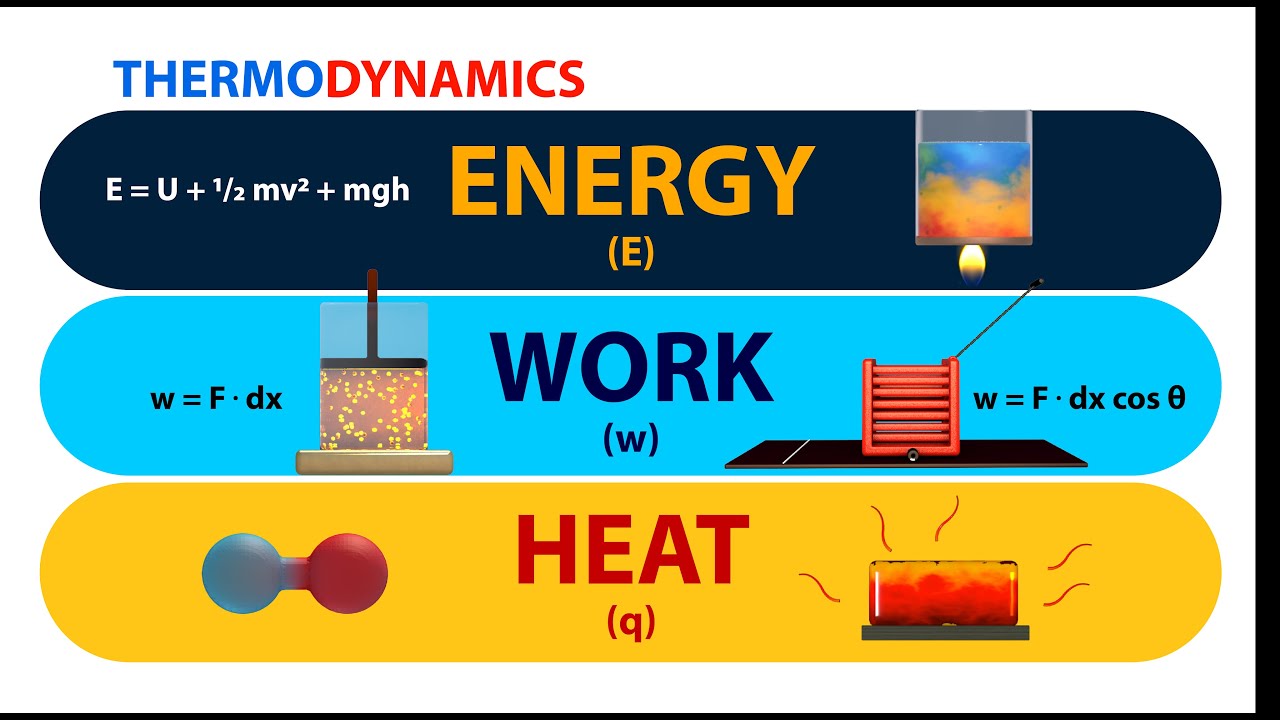
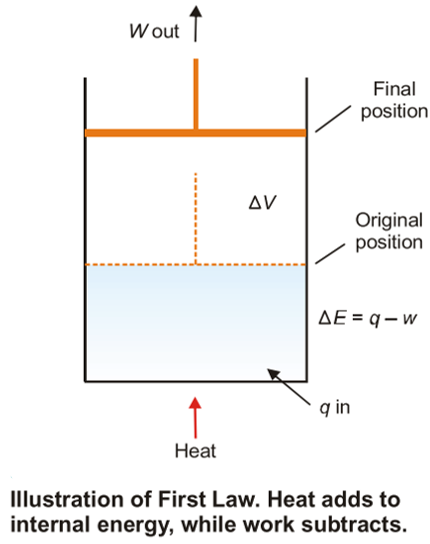
Thermodynamics Energy Work And Heat Animation Youtube Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. the behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities. Thermodynamics is a branch of physics that studies the relationships and conversions between heat and other forms of energy. it examines how energy transformations are governed by certain laws, influencing various physical processes and systems. The first law of thermodynamics states that the change in internal energy of a closed system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. Δ u = q − w. 12.6. The laws of thermodynamics are a set of scientific laws which define a group of physical quantities, such as temperature, energy, and entropy, that characterize thermodynamic systems in thermodynamic equilibrium. the laws also use various parameters for thermodynamic processes, such as thermodynamic work and heat, and establish relationships.
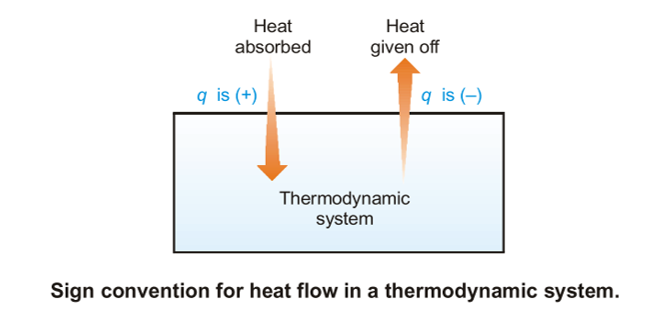
Thermodynamics A Relationships Between Heat And Other Forms Of Energy The first law of thermodynamics states that the change in internal energy of a closed system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. Δ u = q − w. 12.6. The laws of thermodynamics are a set of scientific laws which define a group of physical quantities, such as temperature, energy, and entropy, that characterize thermodynamic systems in thermodynamic equilibrium. the laws also use various parameters for thermodynamic processes, such as thermodynamic work and heat, and establish relationships. Thermodynamics heat, energy, laws: in order to carry through a program of finding the changes in the various thermodynamic functions that accompany reactions—such as entropy, enthalpy, and free energy—it is often useful to know these quantities separately for each of the materials entering into the reaction. for example, if the entropies are known separately for the reactants and. Thermodynamics is the science of the relationship between heat, work, temperature, and energy. the first and second laws were formally stated in works by german physicist rudolf clausius and scottish physicist william thomson about 1860. the third law was developed by german chemist walther nernst from 1906 to 1912.
Thermodynamics A Relationships Between Heat And Other Forms Of Energy Thermodynamics heat, energy, laws: in order to carry through a program of finding the changes in the various thermodynamic functions that accompany reactions—such as entropy, enthalpy, and free energy—it is often useful to know these quantities separately for each of the materials entering into the reaction. for example, if the entropies are known separately for the reactants and. Thermodynamics is the science of the relationship between heat, work, temperature, and energy. the first and second laws were formally stated in works by german physicist rudolf clausius and scottish physicist william thomson about 1860. the third law was developed by german chemist walther nernst from 1906 to 1912.
Thermodynamics A Relationships Between Heat And Other Forms Of Energy

Comments are closed.