The Sulfuric Acid Manufacturing Process
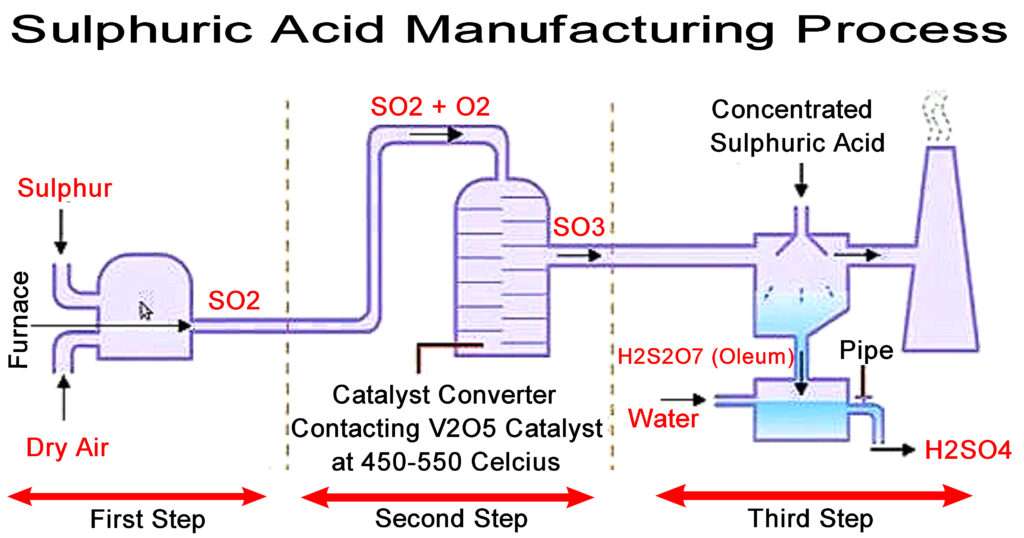
Sulphuric Acid Manufacturing Process Chemical Engineering World Sulphuric acid manufacturing process. sulphuric acid is world’s most produced chemical because of its widespread use in not only in chemical, metallurgical, process, petrochemical, fertilizer industries but also in electrical and electronics, semiconductor industries and also in variety of labs all around the world. Sulfuric acid is a very important commodity chemical; a country's sulfuric acid production is a good indicator of its industrial strength. [9] many methods for its production are known, including the contact process, the wet sulfuric acid process, and the lead chamber process. [10] sulfuric acid is also a key substance in the chemical industry.
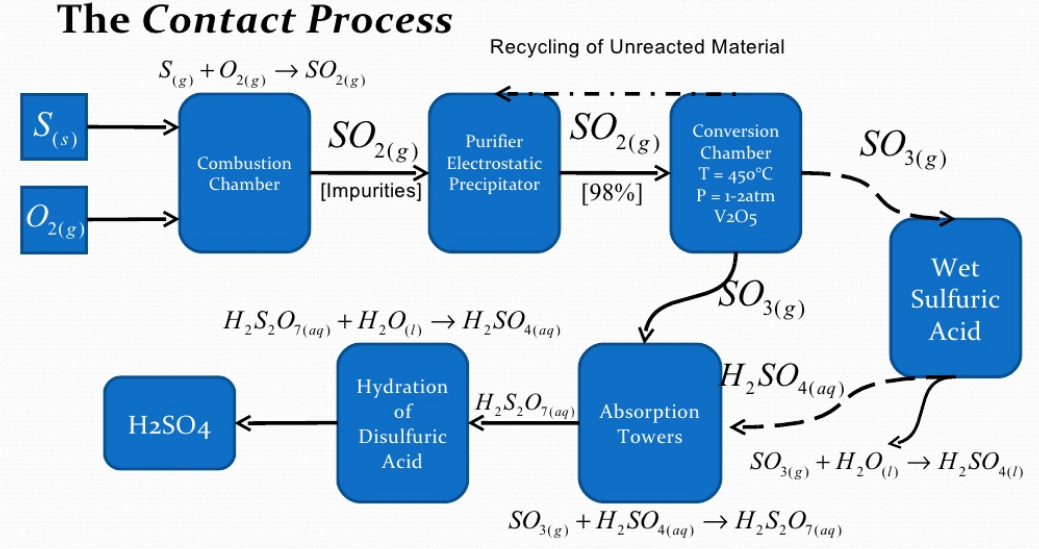
Sulphuric Acid Manufacturing Process The Engineering Concepts As gases, sulfur dioxide (so 2) and sulfur trioxide (so 3) gases are produced. sulfuric acid is manufactured by lead chamber or contact process in industrial scale. sulfur or metal sulfide or hyrogen sulfide gas is burnt with oxygen as the initial step. sulfuric acid has many domestic uses and industrial uses. A schematized overview of some of these interactions is presented in figure 1. chemical industry sulfuric acid, manufacturing, uses: sulfuric acid is by far the largest single product of the chemical industry. the chamber process for its preparation on the scale required by the leblanc process might be regarded as the most important long term. Manufacture of sulphuric acid (h2so4) by contact process. in early days, sulphuric acid used to be maniufactured by lead chamber process. contact process is modern method. the acid obtained is pure( free from impurities) and is quite concentrated (96 99%). the contact process, it’s name is mainly from the fact that the conversion of sulphur. The contact process: makes sulphur dioxide; converts the sulphur dioxide into sulphur trioxide (the reversible reaction at the heart of the process); converts the sulphur trioxide into concentrated sulphuric acid. making the sulphur dioxide. this can either be made by burning sulphur in an excess of air:.
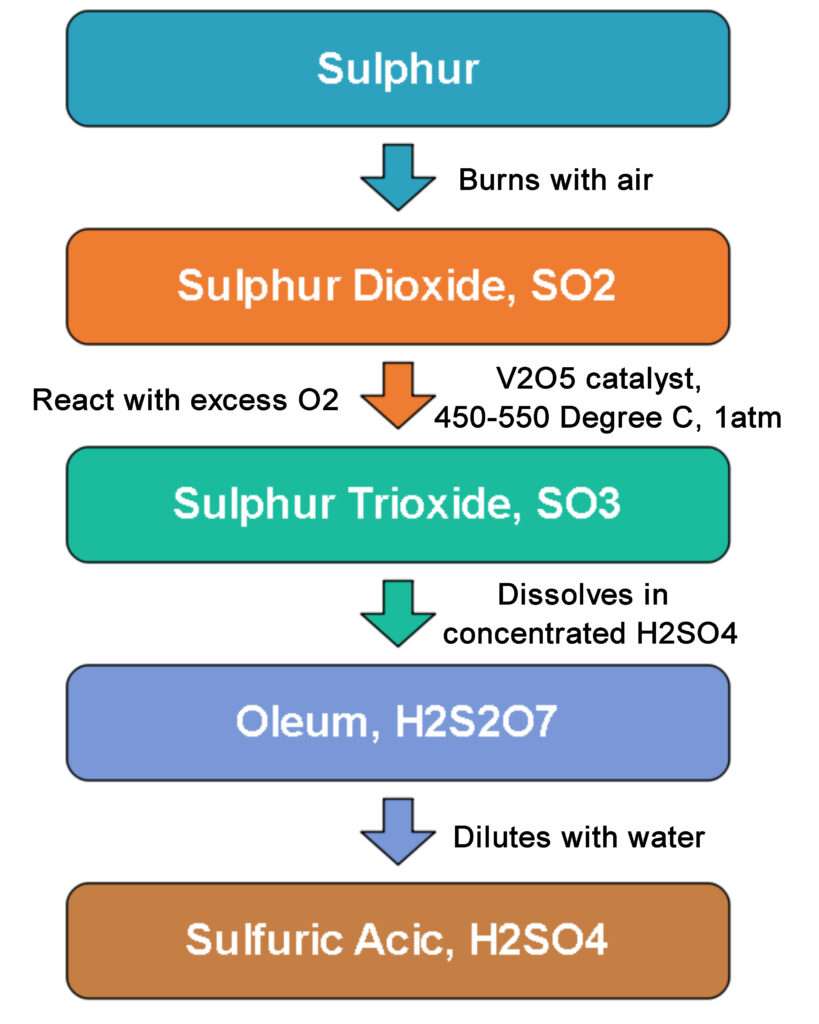
Sulphuric Acid Manufacturing Process Chemical Engineering World Manufacture of sulphuric acid (h2so4) by contact process. in early days, sulphuric acid used to be maniufactured by lead chamber process. contact process is modern method. the acid obtained is pure( free from impurities) and is quite concentrated (96 99%). the contact process, it’s name is mainly from the fact that the conversion of sulphur. The contact process: makes sulphur dioxide; converts the sulphur dioxide into sulphur trioxide (the reversible reaction at the heart of the process); converts the sulphur trioxide into concentrated sulphuric acid. making the sulphur dioxide. this can either be made by burning sulphur in an excess of air:. In this video we briefly look at the four main steps in the industrial manufacture of sulphuric acid. we note down the key reaction equations while explainin. The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic impurities in the sulfur feedstock, vanadium (v) oxide (v 2 o 5) is now preferred.

The Manufacture Of Sulphuric Acid Part 1 Stock Vector Illustration Of In this video we briefly look at the four main steps in the industrial manufacture of sulphuric acid. we note down the key reaction equations while explainin. The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic impurities in the sulfur feedstock, vanadium (v) oxide (v 2 o 5) is now preferred.

Comments are closed.