The Process Of Adiabatic Cooling And Heating
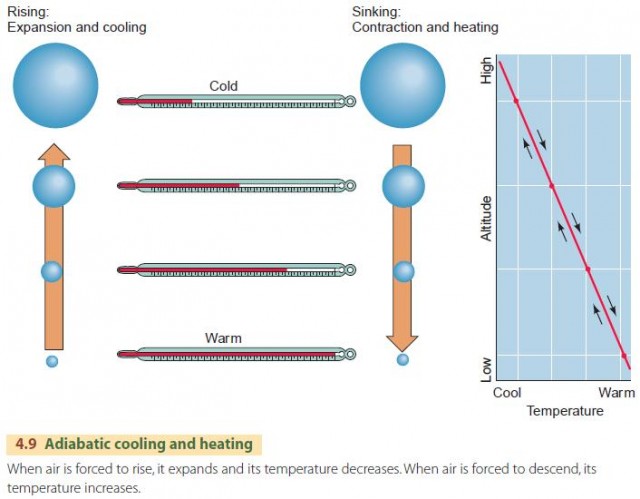
The Adiabatic Process In meteorology, the adiabatic process primarily describes the action of heating or cooling a body of air without any energy exchanged with the surrounding atmosphere. the temperature changes occur as a result of an air pocket's compression or expansion due to pressure changes in the surrounding air. think of the internal combustion engine (ice. Changes in air pressure and temperature cause adiabatic clouds over mountain ranges.exploring the nature of wyoming is produced by:university of wyoming exte.
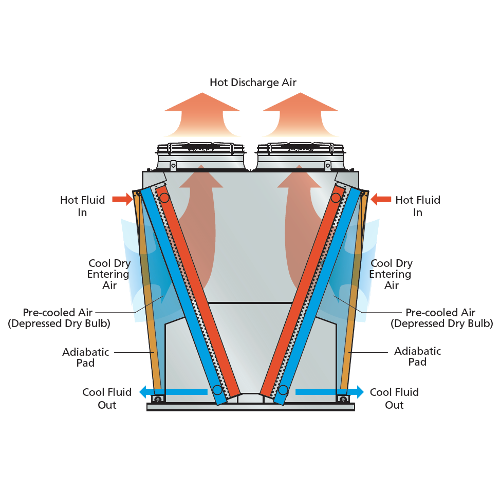
Adiabatic Cooling 101 Evapco Other. category. v. t. e. an adiabatic process (adiabatic from ancient greek ἀδιάβατος (adiábatos) 'impassable') is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. unlike an isothermal process, an adiabatic process transfers energy to the surroundings. Reversible adiabatic process. the reversible adiabatic process is also called an isentropic process. it is an idealized thermodynamic process that is adiabatic and in which the work transfers of the system are frictionless; there is no transfer of heat or of matter, and the process is reversible. such an idealized process is useful in. When a gas is expanded adiabatically, its pressure decreases while its volume increases. it results in a decrease in temperature, a process known as adiabatic cooling. when a gas is compressed adiabatically, its pressure increases, and the volume decreases. as a result, the temperature rises, resulting in a phenomenon known as adiabatic heating. Because we are modeling the process as a quasi static adiabatic compression of an ideal gas, we have pvγ = constant and pv = nrt. the work needed can then be evaluated with w = ∫v2v1pdv. solution. for an adiabatic compression we have p2 = p1(v1 v2)γ, so after the compression, the pressure of the mixture is p2 = (1.00 × 105n m2)(240 × 10.
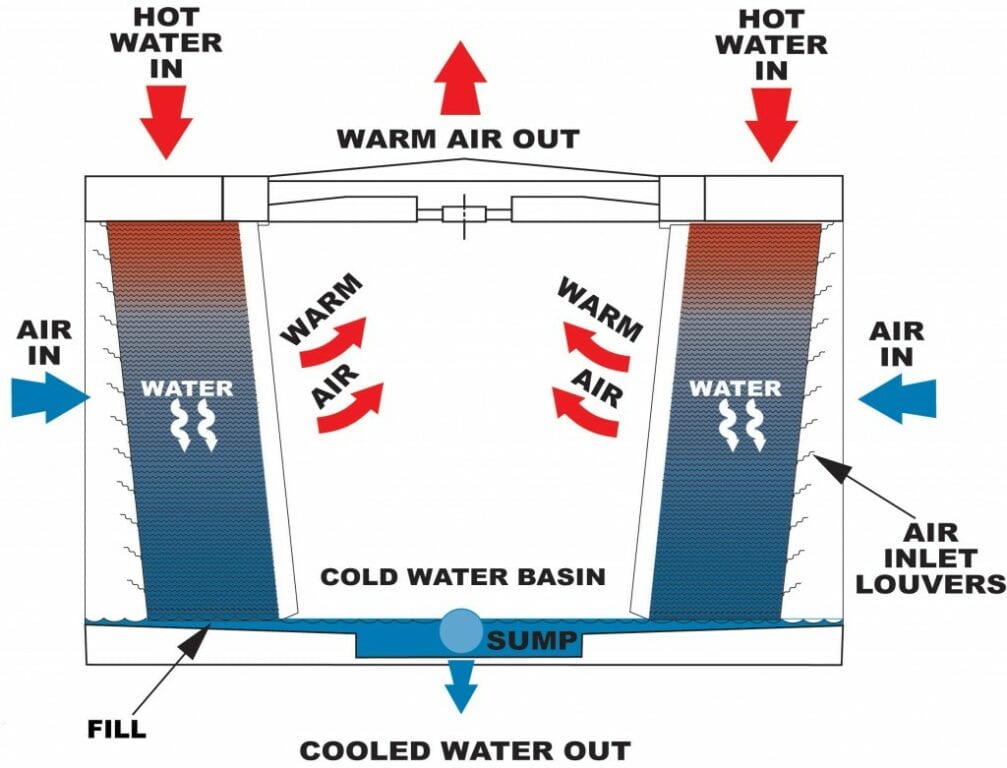
Adiabatic Cooling When a gas is expanded adiabatically, its pressure decreases while its volume increases. it results in a decrease in temperature, a process known as adiabatic cooling. when a gas is compressed adiabatically, its pressure increases, and the volume decreases. as a result, the temperature rises, resulting in a phenomenon known as adiabatic heating. Because we are modeling the process as a quasi static adiabatic compression of an ideal gas, we have pvγ = constant and pv = nrt. the work needed can then be evaluated with w = ∫v2v1pdv. solution. for an adiabatic compression we have p2 = p1(v1 v2)γ, so after the compression, the pressure of the mixture is p2 = (1.00 × 105n m2)(240 × 10. This process is idealized to be adiabatic. adiabatic heating and cooling. adiabatic compression of gas causes an increase in the gas temperature. adiabatic expansion against pressure, or a spring, causes a drop in temperature. in contrast, free expansion is an isothermal process for an ideal gas. Updated on june 20, 2019. in physics, an adiabatic process is a thermodynamic process in which there is no heat transfer into or out of a system and is generally obtained by surrounding the entire system with a strongly insulating material or by carrying out the process so quickly that there is no time for a significant heat transfer to take.

Comments are closed.