The Mole And Avogadros Number Atomic Structure And Properties Ap Chemistry Khan Academy

The Mole And Avogadro S Number Atomic Structure And Properties о Keep going! check out the next lesson and practice what you’re learning: khanacademy.org science ap chemistry beta x2eef969c74e0d802:atomic struct. One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). the number 6.022 × 10²³ is known as avogadro's number or avogadro's constant. the concept of the mole can be used to convert between mass and number of particles.
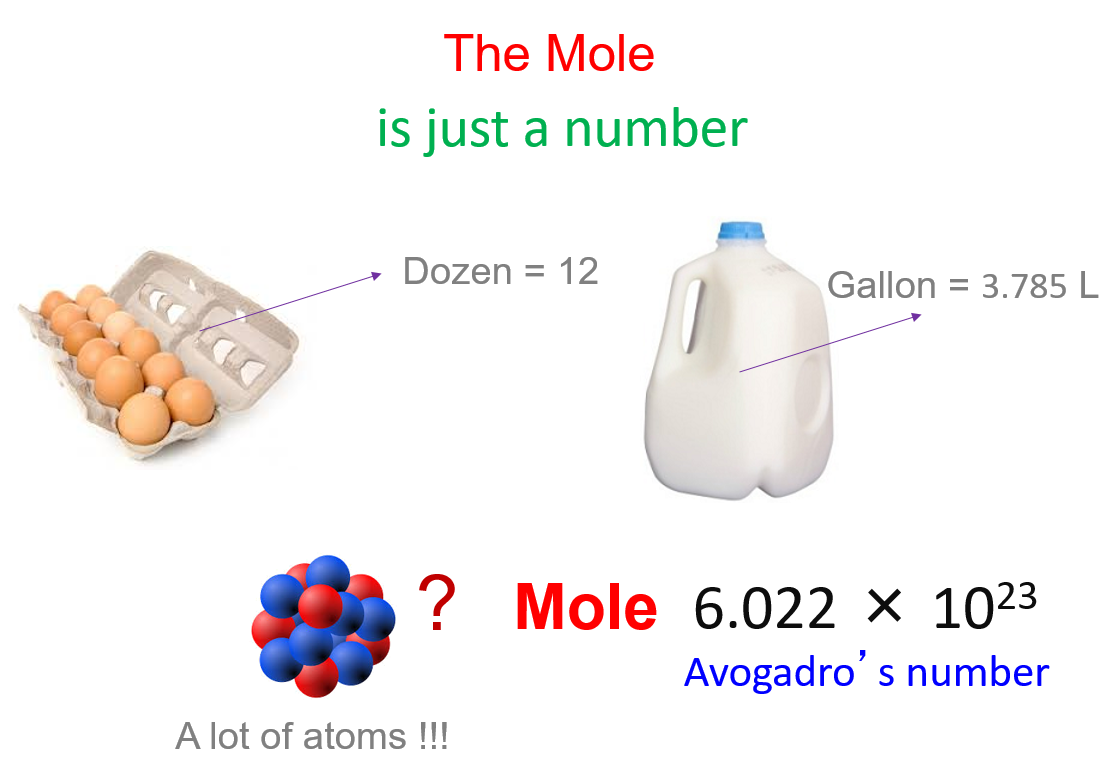
The Mole And Molar Mass Chemistry Steps If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Three types of questions on the ap exam. 1. calculate avg atomic mass from mass spectrum (might be fictitious element) avg am = (abundance of 1st isotope × its atomic mass) (abundance of 2nd isotope × its atomic mass) 100. 2. identify element from mass spectrum. Edexcel. spanish. past papers. cie. spanish language & literature. past papers. other subjects. revision notes on the mole concept for the college board ap chemistry syllabus, written by the chemistry experts at save my exams. The mole (symbol: mol) is defined as the amount of any substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of pure carbon 12 (¹²c). this number is known as avogadro’s number, which is approximately 6.022×10 23. avogadro’s number. avogadro’s number (n a): 6.022×10 23.
.PNG)
Avogadro S Number And The Mole Edexcel. spanish. past papers. cie. spanish language & literature. past papers. other subjects. revision notes on the mole concept for the college board ap chemistry syllabus, written by the chemistry experts at save my exams. The mole (symbol: mol) is defined as the amount of any substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of pure carbon 12 (¹²c). this number is known as avogadro’s number, which is approximately 6.022×10 23. avogadro’s number. avogadro’s number (n a): 6.022×10 23. This general chemistry video tutorial focuses on avogadro's number and how it's used to convert moles to atoms. this video also shows you how to calculate t. If we are given the number of atoms of an element x, we can convert it into moles of by using the relationship. 1 mol x = 6.022 ×1023 x atoms. 1 mol x = 6.022 × 10 23 x atoms. an example on the use of avogadro's number as a conversion factor is given below for carbon.
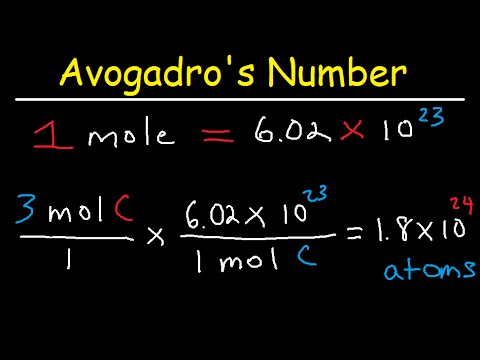
Avogadro S Number And The Mole This general chemistry video tutorial focuses on avogadro's number and how it's used to convert moles to atoms. this video also shows you how to calculate t. If we are given the number of atoms of an element x, we can convert it into moles of by using the relationship. 1 mol x = 6.022 ×1023 x atoms. 1 mol x = 6.022 × 10 23 x atoms. an example on the use of avogadro's number as a conversion factor is given below for carbon.

Comments are closed.