The Equilibrium Phase Diagram A And The Non Equilibrium Phase

Schematic Of An Equilibrium And Non Equilibrium Phase Diagram A And One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.

The Equilibrium Phase Diagram A And The Non Equilibrium Phase The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in. equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. The phase equilibrium diagram is the road map to the use of two (or more) metals. as the result, there have been numerous efforts to compile the existing (reported) phase equilibriums in a book form or electronic form. on the other hand, there is an effort also to theoretically calculate the phase diagrams (society of calphad). Both the equilibrium phase diagram (fig. 1) and the scheil non equilibrium phase diagram (fig. 9) can be used to determine the forming phases in the slow equilibrium and the fast cooling processes.

The Equilibrium Phase Diagram A And The Non Equilibrium Phase The phase equilibrium diagram is the road map to the use of two (or more) metals. as the result, there have been numerous efforts to compile the existing (reported) phase equilibriums in a book form or electronic form. on the other hand, there is an effort also to theoretically calculate the phase diagrams (society of calphad). Both the equilibrium phase diagram (fig. 1) and the scheil non equilibrium phase diagram (fig. 9) can be used to determine the forming phases in the slow equilibrium and the fast cooling processes. Figure 1. a hypothetical binary phase diagram with an isopleth at 32 weight percent b. figure 2. a hypothetical ternary phase diagram with an isopleth at 20% a, 70%b, 10%c. when studying phase diagrams, the most common exercise is an isoplethal analysis. an isopleth* is a line of constant composition, shown in figures 1 and 2. isopleths are. The non crystalline δ phase is demonstrated to be either a metastable transient pre order for crystallization or a thermodynamically stable phase. based on the non equilibrium phase diagrams.
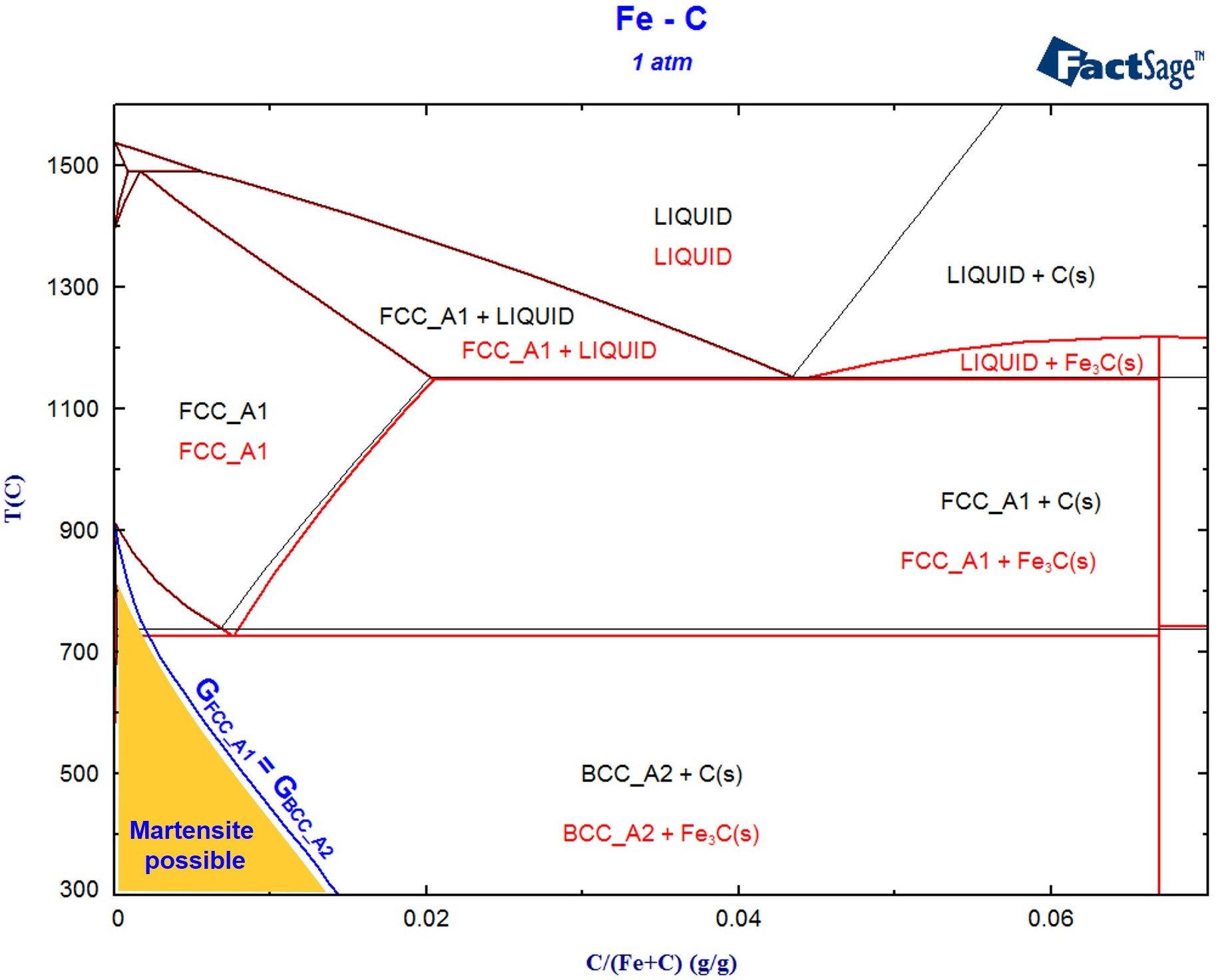
Example For A Factsage Calculation Of A Non Equilibrium Process Gtt Figure 1. a hypothetical binary phase diagram with an isopleth at 32 weight percent b. figure 2. a hypothetical ternary phase diagram with an isopleth at 20% a, 70%b, 10%c. when studying phase diagrams, the most common exercise is an isoplethal analysis. an isopleth* is a line of constant composition, shown in figures 1 and 2. isopleths are. The non crystalline δ phase is demonstrated to be either a metastable transient pre order for crystallization or a thermodynamically stable phase. based on the non equilibrium phase diagrams.

Comments are closed.