The Bond Order Of Co Molecule On The Basis Of Molecular Orbital Theory Is
The Bond Order Of Co Molecule On The Basis Of Molecular Orbital Theory Is Bond order is defined as, the number of bonds formed between the atoms in the compound. we know that molecular orbital theory (mot) explains the formation of molecules, which also helps in determining the bond order of the molecule. the bond order formula derived from mot can be given as: bond order = (no. of bonding electrons no. of. Explain the molecular orbital structure, bond order, stability and magnetic behavior of hydrogen molecule on the basis of molecular orbital theory.
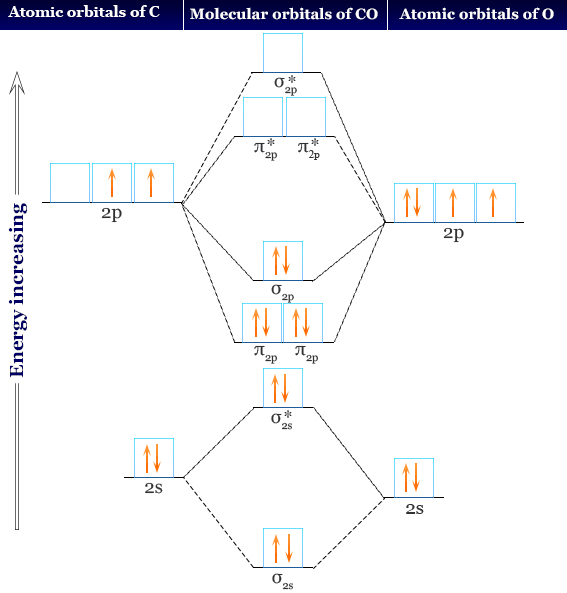
Carbon Monoxide Facts Bonding Properties Uses Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. This page titled 9.8: molecular orbital theory is shared under a cc by 3.0 license and was authored, remixed, and or curated by stephen lower via source content that was edited to the style and standards of the libretexts platform. the molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as. Just as an atomic orbital, whether unhybridized or hybridized, describes a region of space around an atom where an electron is likely to be found, so a molecular orbital describes a region of space in a molecule where electrons are most likely to be found. like an atomic orbital, a molecular orbital has a specific size, shape, and energy. Molecular orbital diagram of he 2. bond order is the number of chemical bonds between a pair of atoms. the bond order of a molecule can be calculated by subtracting the number of electrons in anti bonding orbitals from the number of bonding orbitals, and the resulting number is then divided by two. a molecule is expected to be stable if it has.

Comments are closed.