Tests For Positive Ions 2 Qualitative Tests Learning
Tests For Positive Ions 2 Qualitative Tests Learning 2 learning objectives. by the end of the session: everyone will have experience of carrying out qualitative tests for positive ions. most students will be able to identify aluminium, calcium, copper (ii), iron (ii), iron (iii) ammonium and magnesium ions. a few students will be able to identify an unknown ion using qualitative tests. Iv. chemical test for negative ions. in this investigation you will discover which reagent is best suited to analyze a water sample for presence of anions such as sulfate, chloride and phosphate. available reagents are silver nitrate (agno 3), barium nitrate (ba (no 3) 2) and calcium chloride (cacl 2).

Tests For Positive Ions 2 Qualitative Tests Learning Place 5 6 drops of the na 3 po 4 solution in a test tube. add 1 2 drops of hno 3. warm the test tube in a hot water bath for 5 10 minutes, after the solutions are warmed add 2 drops of ammonium molybdate solution, (nh 4) 2 moo 4. the resulting yellow precipitate indicates the presence of po 43 . record your observations. N goalby chemrevise.org 2 testing for positive ions (c ations) test for ammonium ion nh4 , by reacting with warm naoh(aq ) forming nh3 gas ammonia gas can be identified by its pungent smell or by turning red litmus paper blue precipitation reactions with sodium hydroxide and ammonia the bases oh and ammonia when in limited amounts form the. In this experiment you will determine what ions are present in a solution. to test for an ion, it must first be separated from other similar ions and then tested for. the separations are based on solubility differences. the ions we will be testing for are ag , pb2 , hg2 2 . Other examples of ‘molecular ions’ you will come across are: • the nitrate ion (no 3 ) which has an overall charge of 1 and forms ionic compounds such as sodium nitrate (nano 3) where the nitrate group gains an electron from a sodium atom. • the sulfate ion (so 4 2 ), which has an overall charge of 2 and forms ionic compounds.
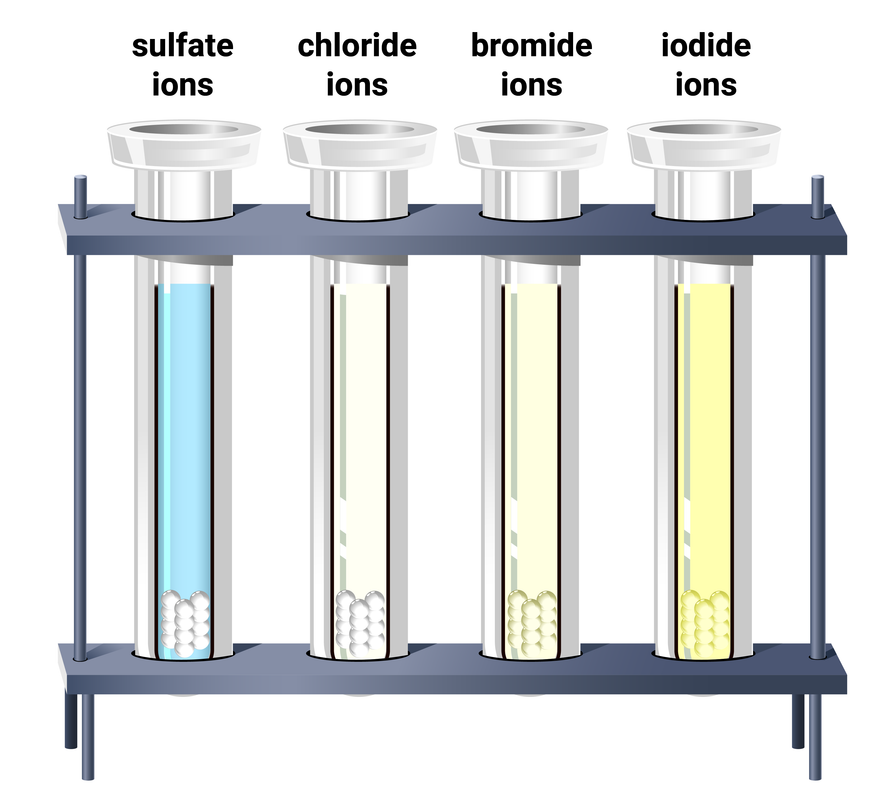
Qualitative Analysis Tests For Ions Edexcel T9 Revisechemistry Uk In this experiment you will determine what ions are present in a solution. to test for an ion, it must first be separated from other similar ions and then tested for. the separations are based on solubility differences. the ions we will be testing for are ag , pb2 , hg2 2 . Other examples of ‘molecular ions’ you will come across are: • the nitrate ion (no 3 ) which has an overall charge of 1 and forms ionic compounds such as sodium nitrate (nano 3) where the nitrate group gains an electron from a sodium atom. • the sulfate ion (so 4 2 ), which has an overall charge of 2 and forms ionic compounds. A common test to distinguish group 1 and group 2 ions is the flame test, where the metal compound or its solution is heated in a roaring blue bunsen flame. the resulting coloured flame can be used to identify the cation present. a number of experimental techniques can be used as shown in the videos below. 9.6c identify the ions in unknown salts, using the tests for the specified cations and anions in 9.2c, 9.3c, 9.4c, 9.5c; topic 9 separate chemistry 2. qualitative analysis: test for ions. 9.2cd calcium ion, ca²⁺ (orange red) 9.2ce copper ion, cu²⁺ (blue green) 9.3cb calcium ion, ca²⁺ 9.4c describe the chemical test for ammonia.

Comments are closed.