Testing For Positive Metal Ions Hydroxide Precipitates Aqa

Testing For Positive Metal Ions Hydroxide Precipitates Aqa Most anions are formed from non metals. from the metal compound close compound a substance formed by the chemical union of two or more elements., eg the sulfate ion (so 4 2 ) if the copper. The blue hydroxide precipitate colour shows that cu 2 ions are present, and the white barium sulfate precipitate shows that so 4 2 ions are present. question identify salt c using the results in.
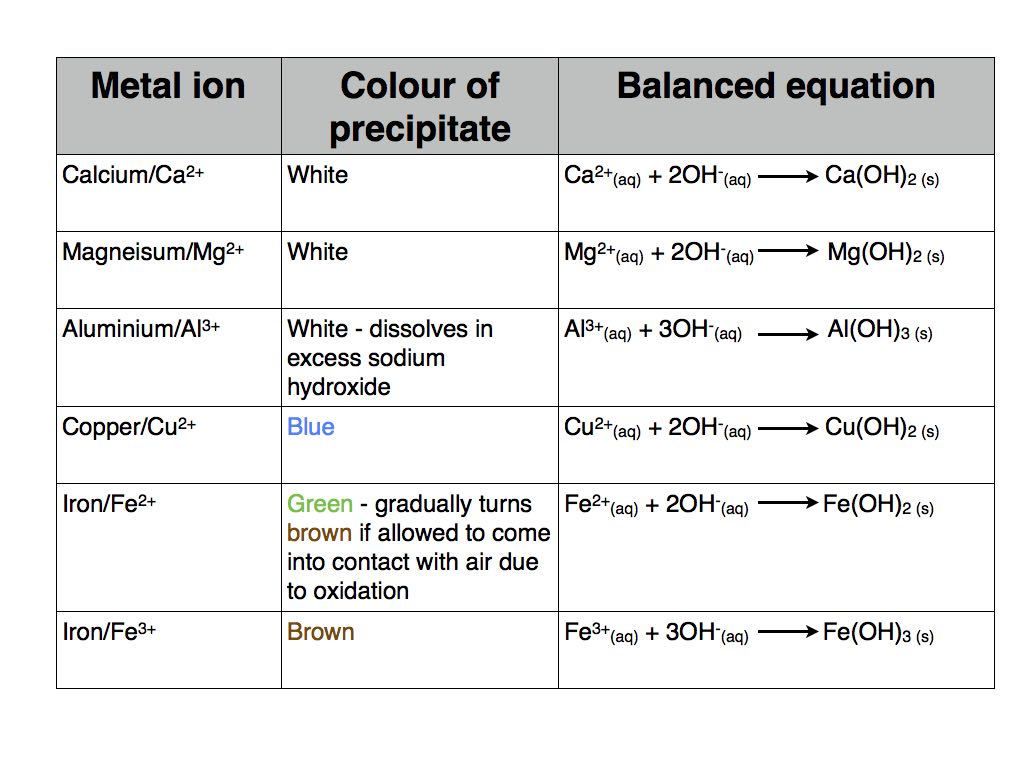
Metal Hydroxide Tests Copy 001 Online Chemistry Tutor About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. A revision video for unit 8 of aqa gcse chemistry (8462) looking at the hydroxide test used to identify metal cations in solutions. this covers specification. Let’s look at the method for the sodium hydroxide test: 1. dissolve a small amount of the substance in water. 2. place about 5cm³ of the sample solution into a test tube. 3. slowly add a few drops of sodium hydroxide solution. avoid adding too much naoh too quickly, as this may dissolve the initial precipitate. 4. Core chemistry 14 16. testing for positive ions. this page looks at tests for a number of positive ions in solution using sodium hydroxide solution and ammonia solution. there is a wide variation between what various syllabuses might want you to know about this, and it is is essential that you find out what level of detail your examiners want.

Comments are closed.