Table 1 From Brentuximab Vedotin In Combination With Rituximab
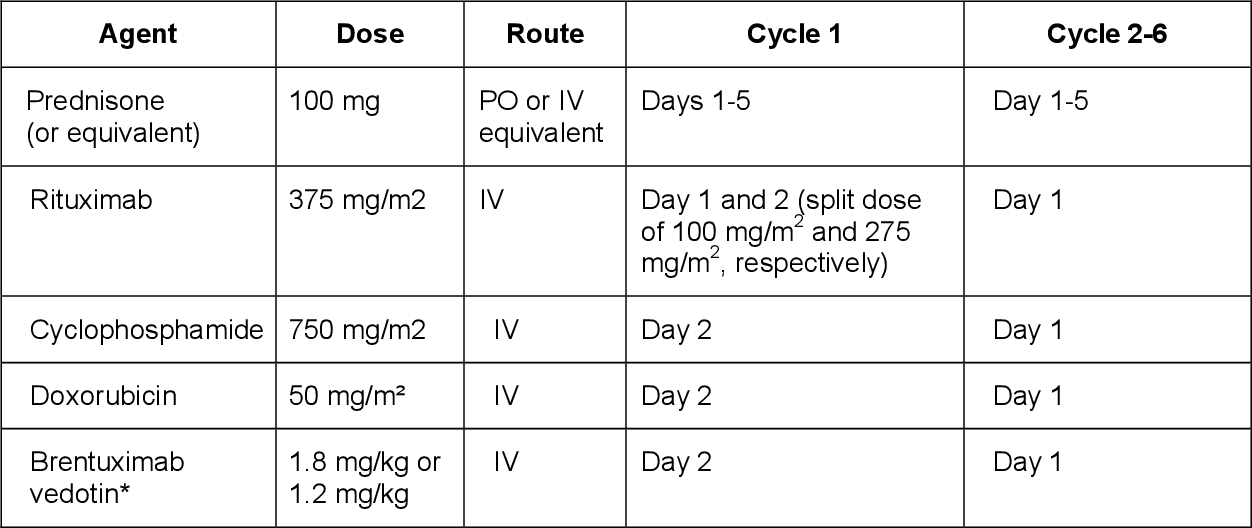
Table 1 From Brentuximab Vedotin In Combination With Rituximab Introduction. brentuximab vedotin (bv) is an immunoconjugate consisting of a cd30 directed antibody linked to the anti microtubule agent auristatin. 1 bv is highly active in relapsed and refractory (r r) classical hodgkin lymphoma and in cd30 expressing t cell lymphomas. 2, 3 in the frontline setting, bv combined with chemotherapy has been recently approved for advanced classical hodgkin. Table 1. the study regimen: brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (bv r chp). the study was conducted in three academic centers in the usa and was run in accordance with the declaration of helsinki.
Brentuximab Vedotin In Combination With Rituximab Cyclophosphamide We conducted a phase i ii multicenter trial using 6 cycles of brentuximab vedotin (bv) in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (r chp) for treatment of patients with cd30 positive ( ) b cell lymphomas. thirty one patients were evaluable for toxicity and 29 for ef …. Table 1: summary of the combination regimen using brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin and prednisone (r chp). "brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with cd30 positive b cell lymphomas". Consolidative radiation fol lowing end of treatment response assessment was permissible and used in 52% of all patients including 59% of the patients with primary medi astinal b cell lymphoma. with a median follow up of 30 months, the 2 year progression free survival and overall survival rates were 85% (95% ci: 66 94) and 100%, respectively. Bv r chp with or without consolidative radiation is a feasible and active frontline regimen for cd30 positive bcell lymphomas. we conducted a phase i ii multicenter trial using six cycles of brentuximab vedotin (bv) in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (r chp) for treatment of patients with cd30 positive b cell lymphomas. thirty one patients were.

Table 1 From Brentuximab Vedotin As Consolidation Therapy After Consolidative radiation fol lowing end of treatment response assessment was permissible and used in 52% of all patients including 59% of the patients with primary medi astinal b cell lymphoma. with a median follow up of 30 months, the 2 year progression free survival and overall survival rates were 85% (95% ci: 66 94) and 100%, respectively. Bv r chp with or without consolidative radiation is a feasible and active frontline regimen for cd30 positive bcell lymphomas. we conducted a phase i ii multicenter trial using six cycles of brentuximab vedotin (bv) in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (r chp) for treatment of patients with cd30 positive b cell lymphomas. thirty one patients were. Subsequently, a further 13 patients with dlbcl were recruited and administered bv (1.8 mg kg) in combination with rituximab (375 mg m 2), yielding an orr of 46%. first line treatment with 1.2 mg kg bv (n=22) or 1.8 mg kg bv (n=29) plus rituximab chop for patients with dlbcl is currently under evaluation in a phase ii study [ 88 ]. A more recent approach has been to add novel agents including brentuximab vedotin and pd 1 blocking antibodies to standard chemotherapy regimens (see table 1). brentuximab vedotin was initially combined with abvd, and subsequently substituted for bleomycin, in a phase i study. 78 in this combination study, complete responses were seen in most.

Table 1 From Brentuximab Vedotin In The Front Line Treatment Of Subsequently, a further 13 patients with dlbcl were recruited and administered bv (1.8 mg kg) in combination with rituximab (375 mg m 2), yielding an orr of 46%. first line treatment with 1.2 mg kg bv (n=22) or 1.8 mg kg bv (n=29) plus rituximab chop for patients with dlbcl is currently under evaluation in a phase ii study [ 88 ]. A more recent approach has been to add novel agents including brentuximab vedotin and pd 1 blocking antibodies to standard chemotherapy regimens (see table 1). brentuximab vedotin was initially combined with abvd, and subsequently substituted for bleomycin, in a phase i study. 78 in this combination study, complete responses were seen in most.

Comments are closed.