System Engineering For A Novel Continuous Pharmaceutical Manufacturing
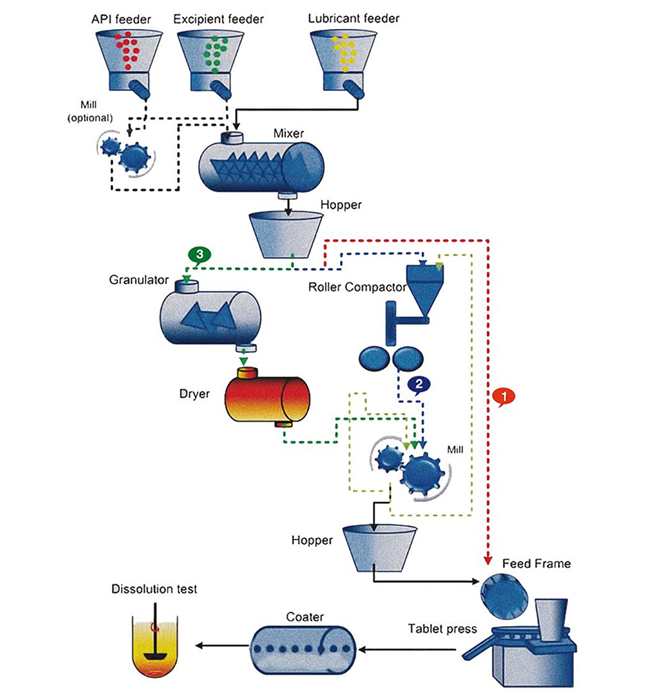
System Engineering For A Novel Continuous Pharmaceutical Manufacturing The continuous pharmaceutical manufacturing process need to be monitored in real time. the main objectives and associated challenges are as follows: (1) identify and integrate different tools needed for real time sensing. (2) identify inbuilt, external available and non available sensors. The objective of this work is to demonstrate the real time advanced model predictive control of a novel continuous direct compaction pharmaceutical tablet manufacturing pilot plant. 1. process and pilot plant description. the snapshot of the pilot plant developed at rutgers along with the control system overview is shown in figure 1 (whole.
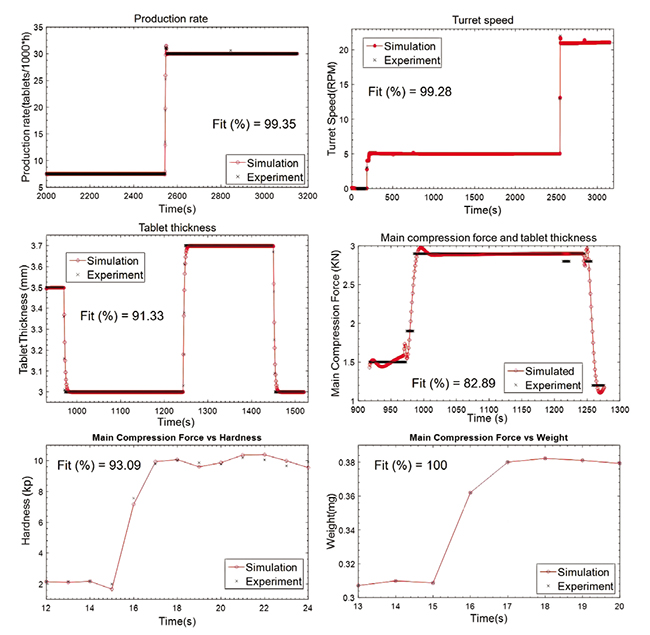
System Engineering For A Novel Continuous Pharmaceutical Manufacturing Eparations processes as well as simplifying the control system design. that is, a research need is to develop both control strategies and design methods for sp. s for continuous pharmaceutical manufacturing.2.3 systems integrationthe control systems for the individual unit operations are coupled to a high. This approach uses a novel modular integrated continuous manufacturing system, which is developed to improve control over process parameters for the purpose of producing pharmaceutical compounds. process intensification is achieved by a holistic integration of novel equipment design such as oscillatory flow crystallizers as well as innovative. Control systems engineering in continuous pharmaceutical manufacturing may 20–21, 2014 continuous manufacturing symposium allan s. myerson,1 markus krumme,2 moheb nasr,3 hayden thomas,4 richard d. braatz1 1massachusetts institute of technology, cambridge, massachusetts 02139 2novartis pharma ag, basel 4056, switzerland. Purpose in pharmaceutical manufacturing, understanding and quantifying how process conditions impact product quality is pivotal to guaranteeing process profitability with sustained product yield. we describe an integrated system model for a commercial scale continuous manufacturing process of a high value active pharmaceutical ingredient (api) and its use to optimize process conditions to.
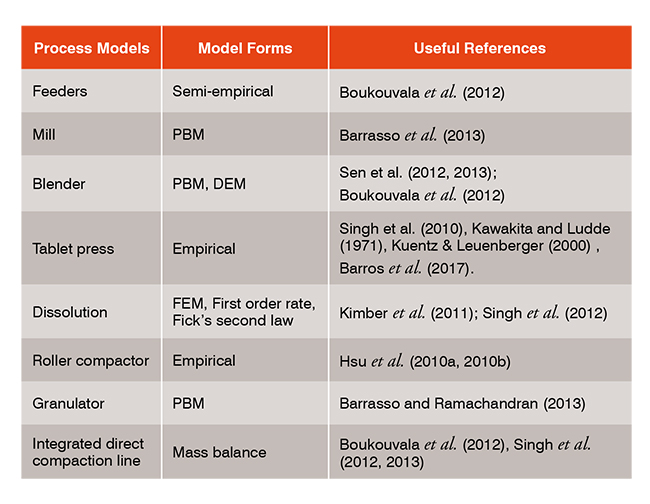
System Engineering For A Novel Continuous Pharmaceutical Manufacturing Control systems engineering in continuous pharmaceutical manufacturing may 20–21, 2014 continuous manufacturing symposium allan s. myerson,1 markus krumme,2 moheb nasr,3 hayden thomas,4 richard d. braatz1 1massachusetts institute of technology, cambridge, massachusetts 02139 2novartis pharma ag, basel 4056, switzerland. Purpose in pharmaceutical manufacturing, understanding and quantifying how process conditions impact product quality is pivotal to guaranteeing process profitability with sustained product yield. we describe an integrated system model for a commercial scale continuous manufacturing process of a high value active pharmaceutical ingredient (api) and its use to optimize process conditions to. Perform and publish economic analysis of benefits of continuous manufacturing. develop integrated control systems to support implementation of feed back or feed forward control. move from end product testing to active control paradigm. develop models for system wide to enable optimization. Currently, pharmaceutical industries are undergoing a paradigm shift from traditional batch to novel continuous manufacturing. few pharmaceutical products have been recently approved by the us food and drug administration (fda) for continuous production, and several others are under evaluation.
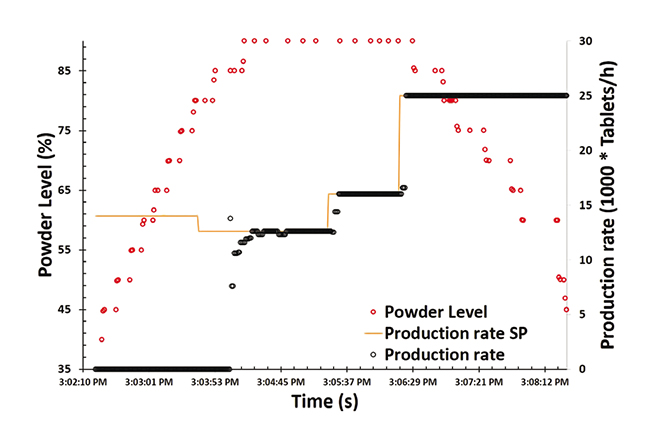
System Engineering For A Novel Continuous Pharmaceutical Manufacturing Perform and publish economic analysis of benefits of continuous manufacturing. develop integrated control systems to support implementation of feed back or feed forward control. move from end product testing to active control paradigm. develop models for system wide to enable optimization. Currently, pharmaceutical industries are undergoing a paradigm shift from traditional batch to novel continuous manufacturing. few pharmaceutical products have been recently approved by the us food and drug administration (fda) for continuous production, and several others are under evaluation.

System Engineering For A Novel Continuous Pharmaceutical Manufacturing

Comments are closed.