Steripath For Blood Cultures

Steripath Gen2 Blood Culture Collection By Magnolia Medical The steripath difference. optimally designed for blood culture contamination prevention. steripath comes pre assembled and sterile to actively divert and sequester the initial 1.5 2.0ml of blood, the volume known to contain contaminants.6 blood cultures are collected through an independent, second flow path, creating a closed vein to bottle collection system designed to prevent bypassing. Steripath micro optimizes the blood culture collection process by allowing for fast, easy, and consistent diversion and test sample collection. using an active blood culture bottle or syringe driven diversion method, steripath micro diverts the initial 0.5 to 1.0 ml of blood into the diversion chamber, preventing potential contaminants from.
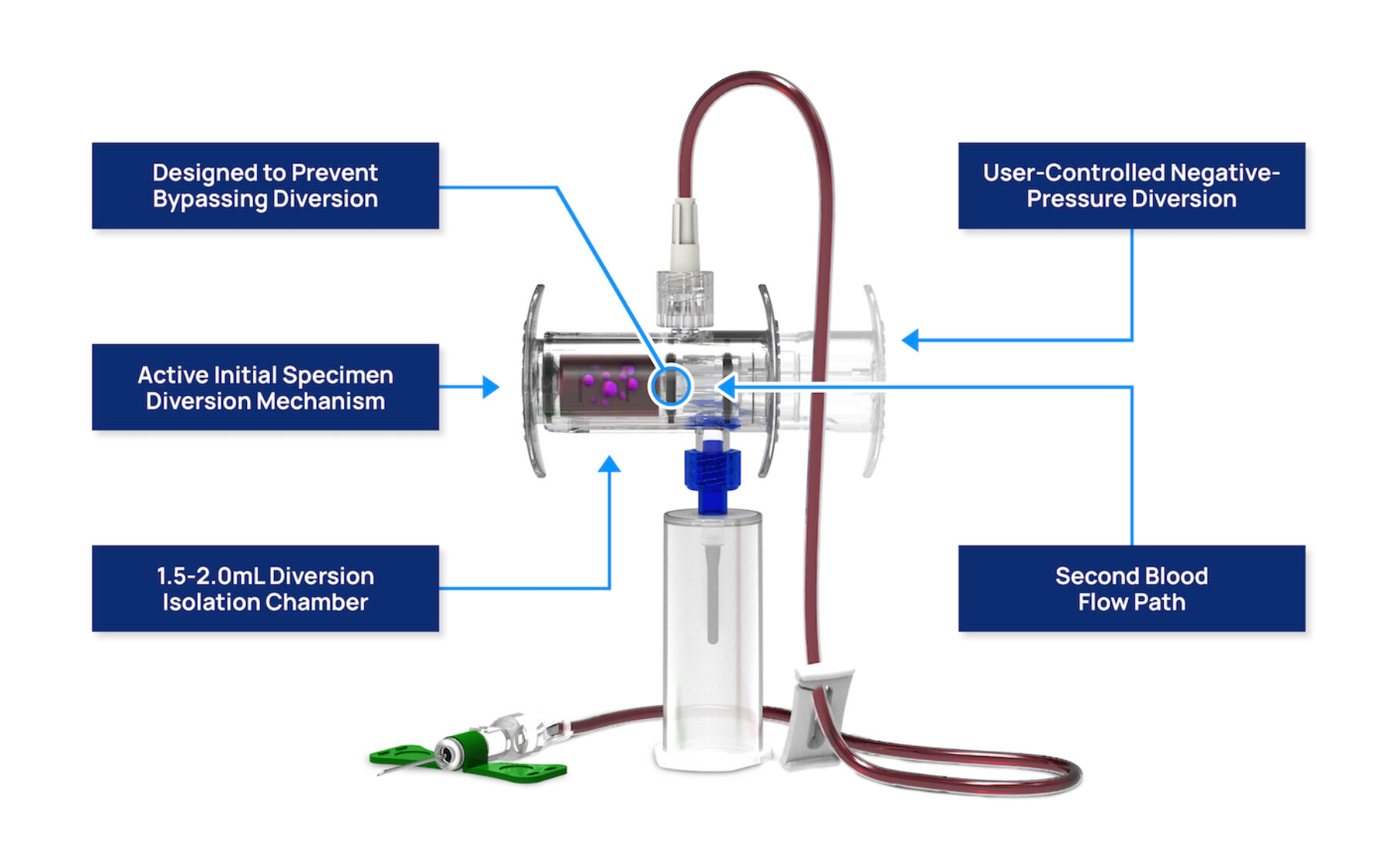
Steripath Blood Culture Collection Reduction in false positive blood culture results by up to 100% 19. 10 month [11,202 blood culture sets] sustained contamination rate as low as 0.0% 19. positive predictive value as high as 97% 20. reduction in vancomycin dot as much as 31% 21. shorten length of stay by average of 2.2 days (p=0.0001) 22. Steripath gen2 is designed with enhanced features to make blood culture collection easy, and to reduce blood culture contamination effectively. this mitigate. A total of 6 studies (5 in the us and 1 in israel) evaluated steripath isdd and found a significant reduction in blood culture contamination to 1% or less. they included 1 randomized clinical trial [ 23 ], 3 prospective controlled nonrandomized studies [ 24–26 ], and 2 quasi experimental [ 27 , 28 ] studies. Steripath by magnolia medical technologies has been clinically proven to virtually eliminate the preventable error of blood culture contamination and false p.

Blood Culture Kit Clinical Lab Products A total of 6 studies (5 in the us and 1 in israel) evaluated steripath isdd and found a significant reduction in blood culture contamination to 1% or less. they included 1 randomized clinical trial [ 23 ], 3 prospective controlled nonrandomized studies [ 24–26 ], and 2 quasi experimental [ 27 , 28 ] studies. Steripath by magnolia medical technologies has been clinically proven to virtually eliminate the preventable error of blood culture contamination and false p. Magnolia medical's flagship product family, steripath, was designed and developed to enable hospitals to dramatically reduce blood culture contamination. steripath is supported by extensive. Steripath® is the only fda 510(k) cleared device platform specifically indicated to reduce blood culture contamination for sepsis testing accuracy. i the steripath® initial specimen diversion devices divert and sequester the initial 1.5 to 2 ml of potentially contaminated blood from the sample and then collect blood for blood cultures.
Steripath Blood Culture Collection Magnolia medical's flagship product family, steripath, was designed and developed to enable hospitals to dramatically reduce blood culture contamination. steripath is supported by extensive. Steripath® is the only fda 510(k) cleared device platform specifically indicated to reduce blood culture contamination for sepsis testing accuracy. i the steripath® initial specimen diversion devices divert and sequester the initial 1.5 to 2 ml of potentially contaminated blood from the sample and then collect blood for blood cultures.

Comments are closed.