Solved Using An Integrated Rate Law For A First Order Chegg
Solved The First Order Integrated Rate Law For A Reaction A C Using an integrated rate law for a first order reactionconsider this reaction:2so3(g)→2so2(g) o2(g)at a certain temperature it obeys this rate law. rate =(9.11×10 4s 1)[so3]reaction is important.round your answer to 2 significant digits. Using an integrated rate law for a first order reaction consider this reaction 2h,po.(ay) p,03(aq) 3h,0 (aq) at a certain temperature it obeys this rate law. rate= (0,0854 s [h, po ] suppose a vessel contains h, po, a concentration of 1.49 m. calculate the concentration of h, po, in the vessel 7.70 seconds later.

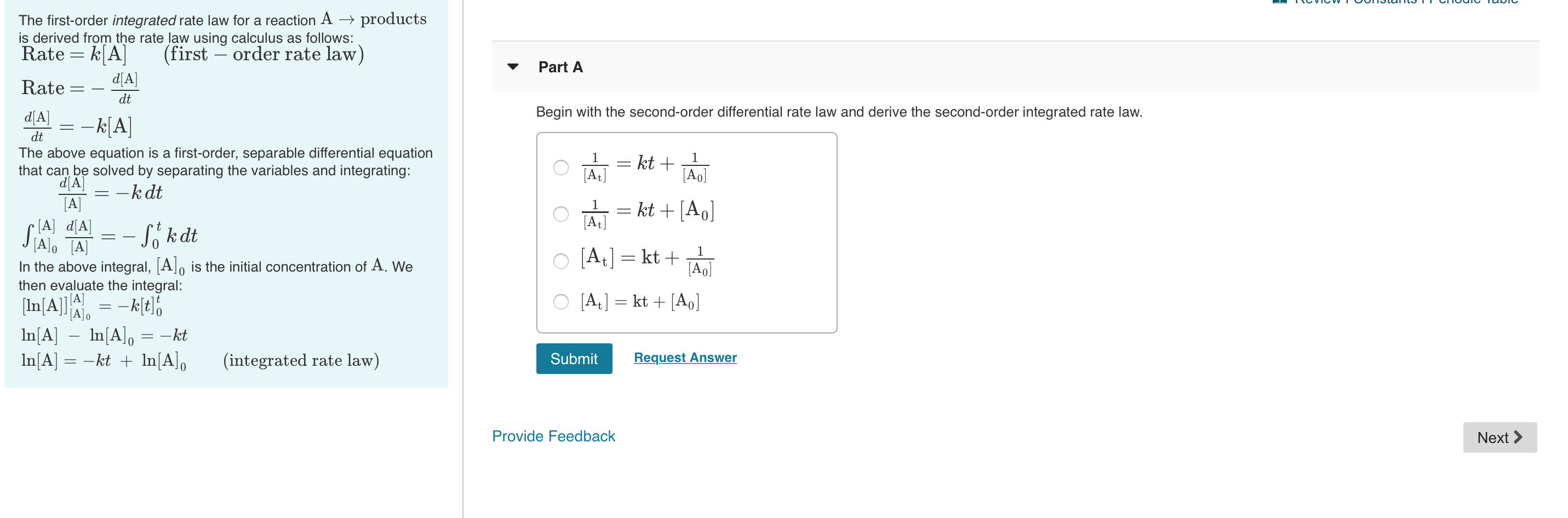
Solved Using First And Second Order Integrated Rate Laws A Using an integrated rate law for a first order reaction consider this reaction: 201,0, (e) 2012 (8) 50, at a certain temperature it obeys this rate law. rate = (0.00120 ')[0,0] suppose a vessel contains ci,o, at a concentration of 0.100 m. calculate the concentration of ci,o, in the vessel 830. seconds later. For purposes of discussion, we will focus on the resulting integrated rate laws for first , second , and zero order reactions. first order reactions. integration of the rate law for a simple first order reaction (rate = k[a]) results in an equation describing how the reactant concentration varies with time:. Integration of the rate law for a simple first order reaction (rate = k[a]) results in an equation describing how the reactant concentration varies with time: \[[a] t=[a] 0 e^{ k t} \nonumber \] where [a]t is the concentration of a at any time t, [a] 0 is the initial concentration of a, and k is the first order rate constant. Table \(\pageindex{1}\) gives the solutions to the integrated rate laws, and you need to know these solutions for zero, first and second order reactions. note, there is a form of each order of reaction that follows the equation of a straight line (y=mx b).

Solved Using First And Second Order Integrated Rate Laws A Integration of the rate law for a simple first order reaction (rate = k[a]) results in an equation describing how the reactant concentration varies with time: \[[a] t=[a] 0 e^{ k t} \nonumber \] where [a]t is the concentration of a at any time t, [a] 0 is the initial concentration of a, and k is the first order rate constant. Table \(\pageindex{1}\) gives the solutions to the integrated rate laws, and you need to know these solutions for zero, first and second order reactions. note, there is a form of each order of reaction that follows the equation of a straight line (y=mx b). Example 12.5.3 12.5. 3: the integrated rate law for a second order reaction. the reaction of butadiene gas (c 4 h 6) with itself produces c 8 h 12 gas as follows: 2c4h6(g) c8h12(g) 2 c 4 h 6 (g) c 8 h 12 (g) the reaction is second order with a rate constant equal to 5.76 × 10 −2 l mol min under certain conditions. The common integrated rate laws. for a zero order reaction: a products , rate = k. the integrated rate law is [a] = kt [a o] for a first order reaction: a products , rate = k [a] the integrated rate law is ln [a] = kt ln [a o] for a second order reaction: 2a products or a b products (when [a] = [b]) , rate = k [a] 2.

Comments are closed.