Solved Molar Concentration Of Fe No3 3 Stock Solution 5 00m C
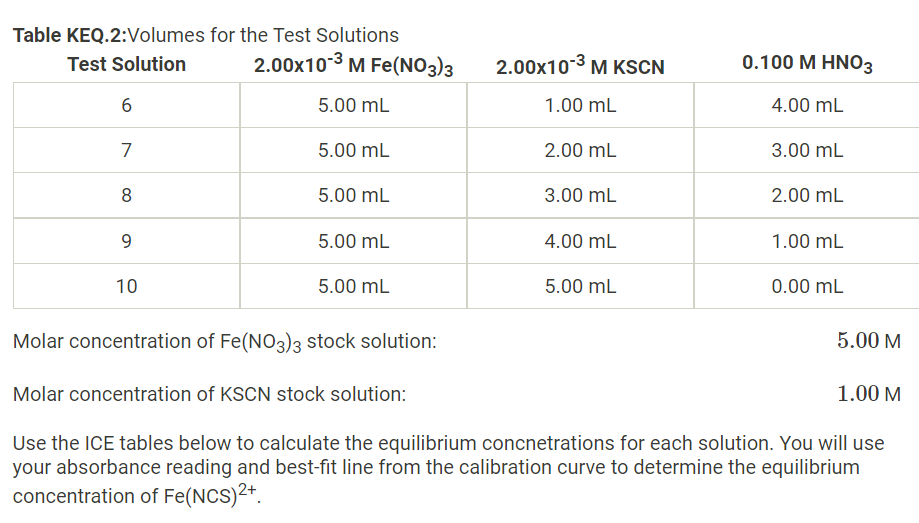
Solved Molar Concentration Of Fe No3 3 Stock Solutionођ Question: molar concentration of fe (no3)3 stock solution: 5.00m molar concentration of kscn stock solution: 1.00m use the ice tables below to calculate the equilibrium concnetrations for each solution. you will use your absorbance reading and best fit line from the calibration curve to determine the equilibrium concentration of fe (ncs)2 use. Module 4: chemical equilibrium calculations data analysis part 1 (data table 1 calculations) to calculate [fe 3 ] ≈ [scn 2 ]: you will use the dilution formula m 1 v 1 = m 2 v 2 where m 1 is the initial molarity of a solution. v 1 is the volume used from the original solution. m 2 is the molarity of the new, diluted, final solution.
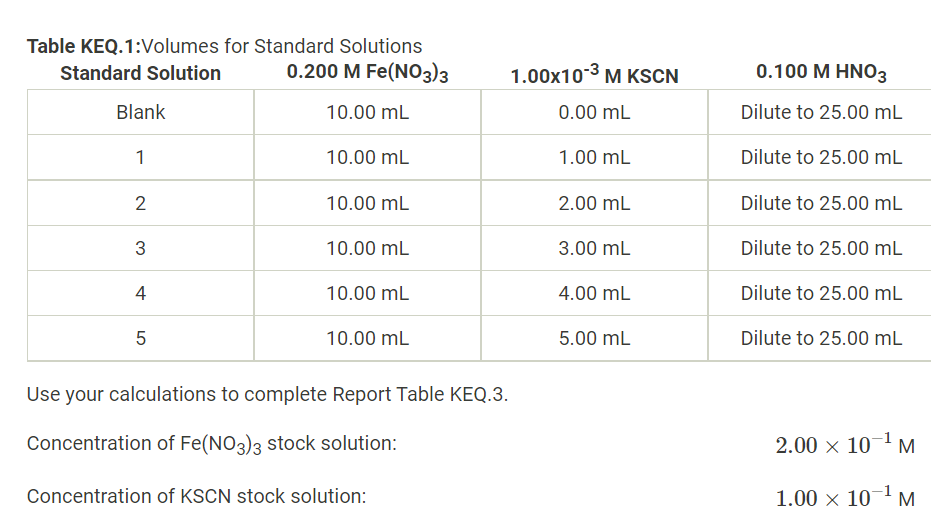
Solved Molar Concentration Of Fe No3 3 Stock Solutionођ Chemical engineering questions and answers. (38pts) b. determining the equilibrium constant recall that the test solutions were prepared according to table keq.2. molar concentration of fe (no3)3 stock solution: 5.00m molar concentration of kscn stock solution: 1.00m use the ice tables below to calculate the equilibrium concnetrations for each. This online calculator can calculate the molar concentration of a solute in a solution, or mass of a solute in a solution with a specific molar concentration. this calculator can solve problems on the molarity or molar concentration of a solute in a solution. first, it can calculate the molar concentration of a solute given a solute chemical. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. you can easily calculate this value, using the following dilution formula: m 1\cdot v 1 = m 2\cdot v 2 m1 ⋅ v 1 = m2 ⋅ v 2. where: m 1. m 1 m1 . The molar mass of cocl 2 •2h 2 o is 165.87 g mol. therefore, molescocl2 ⋅ 2h2o = (10.0g 165.87g mol) = 0.0603mol. the volume of the solution in liters is. volume = 500 ml(1l 1000ml) = 0.500l. molarity is the number of moles of solute per liter of solution, so the molarity of the solution is.
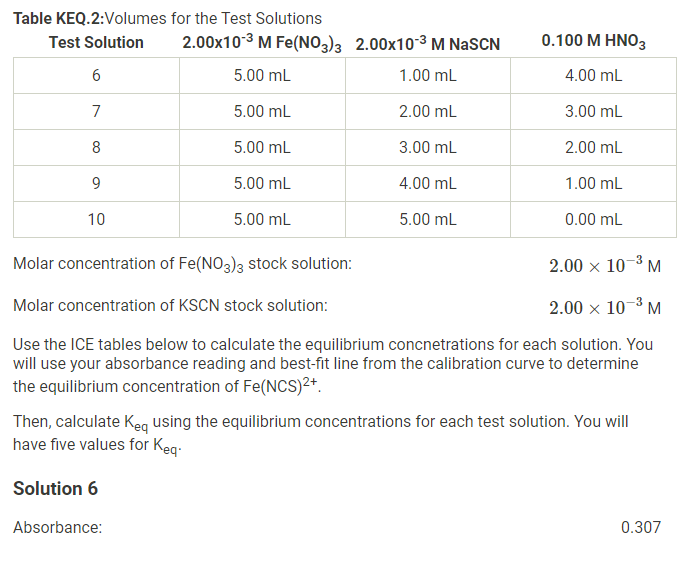
Solved Molar Concentration Of Fe No3 3 Stock Solutionођ You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. you can easily calculate this value, using the following dilution formula: m 1\cdot v 1 = m 2\cdot v 2 m1 ⋅ v 1 = m2 ⋅ v 2. where: m 1. m 1 m1 . The molar mass of cocl 2 •2h 2 o is 165.87 g mol. therefore, molescocl2 ⋅ 2h2o = (10.0g 165.87g mol) = 0.0603mol. the volume of the solution in liters is. volume = 500 ml(1l 1000ml) = 0.500l. molarity is the number of moles of solute per liter of solution, so the molarity of the solution is. C is the molar concentration in mol l (molar or m). this is also referred to as molarity, which is the most common method of expressing the concentration of a solute in a solution. molarity is defined as the number of moles of solute dissolved per liter of solution (mol l = m). a 1 m solution is one in which exactly 1 mole of solute is. Problem #5: a 40.0 ml volume of 1.80 m fe(no 3) 3 is mixed with 21.5 ml of 0.808m fe(no 3) 3 solution. calculate the molar concentration of the final solution. solution: let's use a slightly different way to write the subscripts: m 1 v 1 m 2 v 2 = m 3 v 3. there is no standard way to write the subscripts in problems of this type. substituting:.

Solved Molar Concentration Of Fe No3 3 Stock Solutionођ C is the molar concentration in mol l (molar or m). this is also referred to as molarity, which is the most common method of expressing the concentration of a solute in a solution. molarity is defined as the number of moles of solute dissolved per liter of solution (mol l = m). a 1 m solution is one in which exactly 1 mole of solute is. Problem #5: a 40.0 ml volume of 1.80 m fe(no 3) 3 is mixed with 21.5 ml of 0.808m fe(no 3) 3 solution. calculate the molar concentration of the final solution. solution: let's use a slightly different way to write the subscripts: m 1 v 1 m 2 v 2 = m 3 v 3. there is no standard way to write the subscripts in problems of this type. substituting:.

Comments are closed.