Solved Constants Periodic Table The Integrated Rate Laws F

Solved Using Integrated Rate Laws Constants Periodic Table Question: constants | periodic table the integrated rate laws for zero , first , and second order reaction may be arranged such that they resemble the equation for a straight line,y mx b order integrated rate law graph slope 0 [a], kt [a]0 [a]t vs. t k 1vs.t k a]t 2 al alo. show transcribed image text. here’s the best way to solve it. Example 12.5.3 12.5. 3: the integrated rate law for a second order reaction. the reaction of butadiene gas (c 4 h 6) with itself produces c 8 h 12 gas as follows: 2c4h6(g) c8h12(g) 2 c 4 h 6 (g) c 8 h 12 (g) the reaction is second order with a rate constant equal to 5.76 × 10 −2 l mol min under certain conditions.
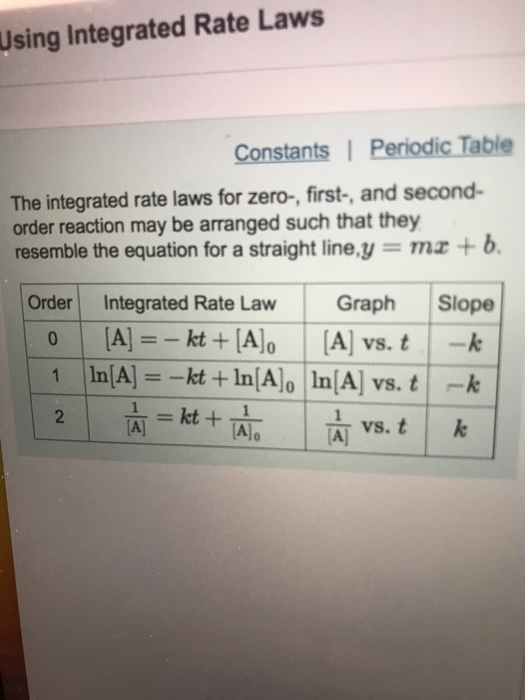
Solved Using Integrated Rate Laws Constants Periodic Table The integrated rate law for zero order kinetics describes a linear plot of reactant concentration, [a] t, versus time, t, with a slope equal to the negative of the rate constant, −k. following the mathematical approach of previous examples, the slope of the linear data plot (for decomposition on w) is estimated from the graph. The integrated rate law for zero order kinetics describes a linear plot of reactant concentration, [a] t, versus time, t, with a slope equal to the negative of the rate constant, −k. following the mathematical approach of previous examples, the slope of the linear data plot (for decomposition on w) is estimated from the graph. Constants periodic table the integrated rate laws for zero , first , and second order reaction may be arranged such that they resemble the equation for a straight line, y=mx b. part c slope k the reactant concentration in a first order reaction was 6.10x10 2 m after 30.0 s and 4.00x10 3 m after 60.0 s. Integrated rate laws. the primary purpose of the integrated rate laws is that they allow us to calculate concentration changes over time. each equation is specific to its order so the order of a reactant must be known before one can calculate its change in concentration over time. there are four variables in the equation: [a], [a]0, k, and t.
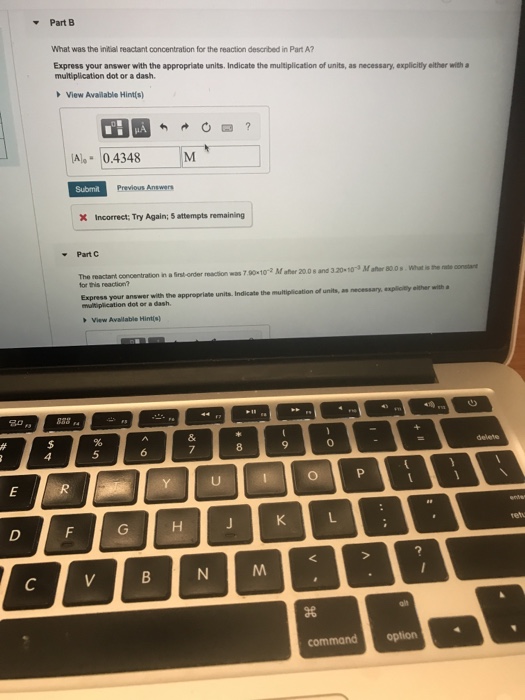
Solved Constants Periodic Table The Integrated Rate Laws C Constants periodic table the integrated rate laws for zero , first , and second order reaction may be arranged such that they resemble the equation for a straight line, y=mx b. part c slope k the reactant concentration in a first order reaction was 6.10x10 2 m after 30.0 s and 4.00x10 3 m after 60.0 s. Integrated rate laws. the primary purpose of the integrated rate laws is that they allow us to calculate concentration changes over time. each equation is specific to its order so the order of a reactant must be known before one can calculate its change in concentration over time. there are four variables in the equation: [a], [a]0, k, and t. Explain the form and function of an integrated rate law. perform integrated rate law calculations for zero , first , and second order reactions. define half life and carry out related calculations. identify the order of a reaction from concentration time data. the rate laws we have seen thus far relate the rate and the concentrations of reactants. Constants| periodic table the integrated rate laws for zero , first , and second order reaction may be arranged such that they resemble the equation for a straight line,y mx b order integrated rate law graph slope vs. 2 vs. part d the reactant concentration in a second order reaction was 0.890 m after 100 s and 8.20x10 2 m after 775 s.
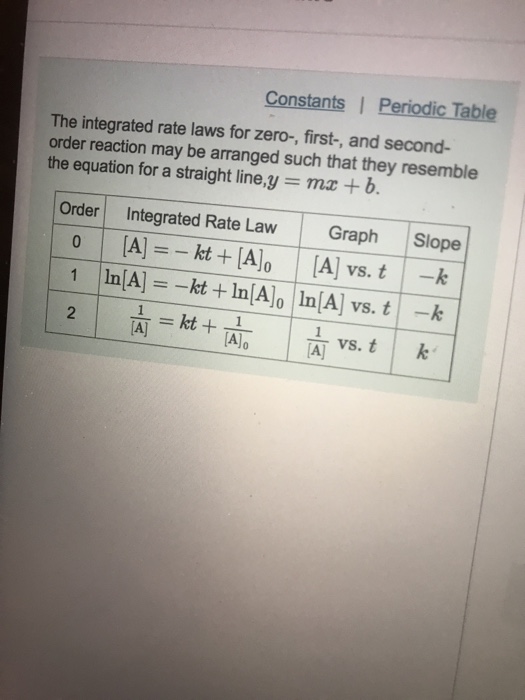
Solved Constants Periodic Table The Integrated Rate Laws C Explain the form and function of an integrated rate law. perform integrated rate law calculations for zero , first , and second order reactions. define half life and carry out related calculations. identify the order of a reaction from concentration time data. the rate laws we have seen thus far relate the rate and the concentrations of reactants. Constants| periodic table the integrated rate laws for zero , first , and second order reaction may be arranged such that they resemble the equation for a straight line,y mx b order integrated rate law graph slope vs. 2 vs. part d the reactant concentration in a second order reaction was 0.890 m after 100 s and 8.20x10 2 m after 775 s.

Comments are closed.