Solved Consider The Reaction Cuo S H2 G в Cu S H2

Solved Consider The Reaction Cuo S H2 G Cu S C In this question, a chemical reaction is given. consider the reaction cuo (s) h2 (g) → cu (s) h20 (1 in this reaction, which substances are the oxidizing agent and reducing agent, respectively? multiple choice cuo and cu h20 and h2 cuo and h2 o ooo o cu and h20 h2 and cuo. Consider the reaction cuo (s) h 2 ( g) → cu (s) h 2 o (l) in this reaction, the oxidant and reductant are, respectively cuo and h 2 h 2 and cuo cuo and cu h 2 o and h 2 none of the above question 2 given the following notation for an electrochemical cell pt (s) ∣ h 2 ( g) ∣ h (aq) ∣∣ ag (aq) ∣ ag (s) 2 h (aq) 2 ag (aq.
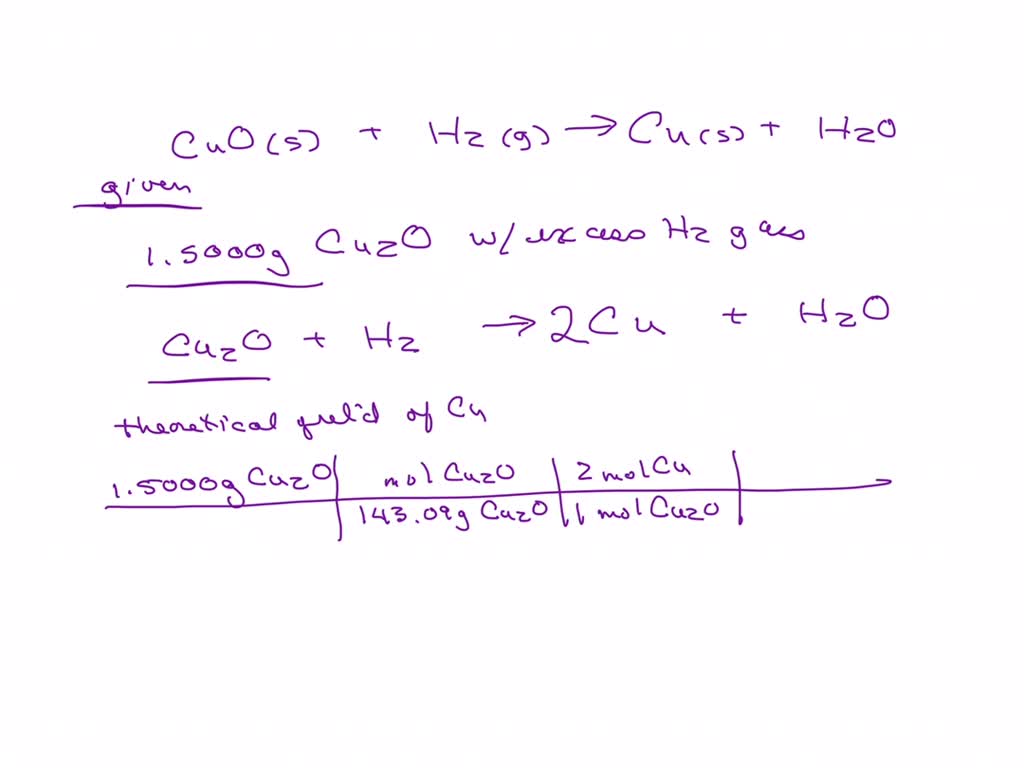
Solved Cuo S H2 G Cu S H2o Suppose You Run The Reaction Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 cuo 2 h2 = cu2 2 h2o. reactants. products. Balancing step by step using the inspection method. let's balance this equation using the inspection method. first, we set all coefficients to 1: 1 h 2 (g) 1 cuo (s) = 1 cu (s) 1 h 2 o (g) for each element, we check if the number of atoms is balanced on both sides of the equation. h is balanced: 2 atoms in reagents and 2 atoms in products. Complete and balance the following redox equations using the smallest whole number coefficients. the reaction takes place in an acidic solution.h2s no3− → s no in the balanced equation, 1 molecules of water are present on the product side. Question: please consider the following reaction: cuo(s) h2(g) < > h2o(g) cu(s) when cuo is added to the equilibrium reaction, will the keq increase, will the key decrease or there will be no change? o a decrease b. no change cincrease.
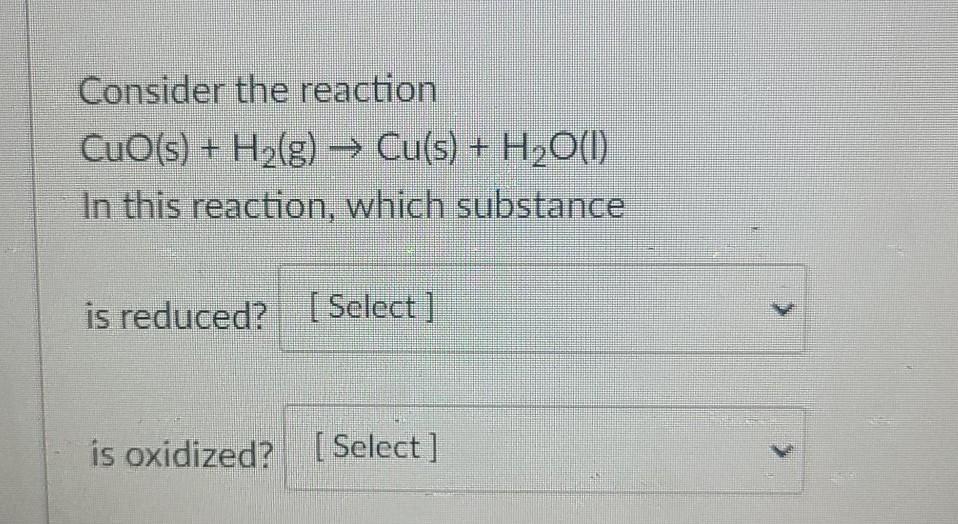
Solved Consider The Reaction Cuo S H2 G в Cu S Complete and balance the following redox equations using the smallest whole number coefficients. the reaction takes place in an acidic solution.h2s no3− → s no in the balanced equation, 1 molecules of water are present on the product side. Question: please consider the following reaction: cuo(s) h2(g) < > h2o(g) cu(s) when cuo is added to the equilibrium reaction, will the keq increase, will the key decrease or there will be no change? o a decrease b. no change cincrease. Study with quizlet and memorize flashcards containing terms like what is the oxidizing agent in the (unbalanced) reaction? cu(s) h (aq) no3 (aq) → no(g) h2o(l) cu2 (aq), consider the reaction cuo(s) h2(g) → cu(s) h2o(l) in this reaction, which substances are the oxidizing agent and reducing agent, respectively?, what is the name given to the experimental apparatus for. Consider the reaction cuo(s) h2(g) cu(s) h20(1). in this reaction, which substances are the oxidizing agent and reducing agent, respectively? cuo and h2 h2 and cuo h2o and h2 cuo and cu none of these choices is correct. true or false: a salt bridge allows charge to build up in an electrochemical cell.

Solved 4 Consider The Reaction Cuo S H2 G Cu S Study with quizlet and memorize flashcards containing terms like what is the oxidizing agent in the (unbalanced) reaction? cu(s) h (aq) no3 (aq) → no(g) h2o(l) cu2 (aq), consider the reaction cuo(s) h2(g) → cu(s) h2o(l) in this reaction, which substances are the oxidizing agent and reducing agent, respectively?, what is the name given to the experimental apparatus for. Consider the reaction cuo(s) h2(g) cu(s) h20(1). in this reaction, which substances are the oxidizing agent and reducing agent, respectively? cuo and h2 h2 and cuo h2o and h2 cuo and cu none of these choices is correct. true or false: a salt bridge allows charge to build up in an electrochemical cell.

Comments are closed.