Solved 5 Draw Two Resonance Structures For The Species Che
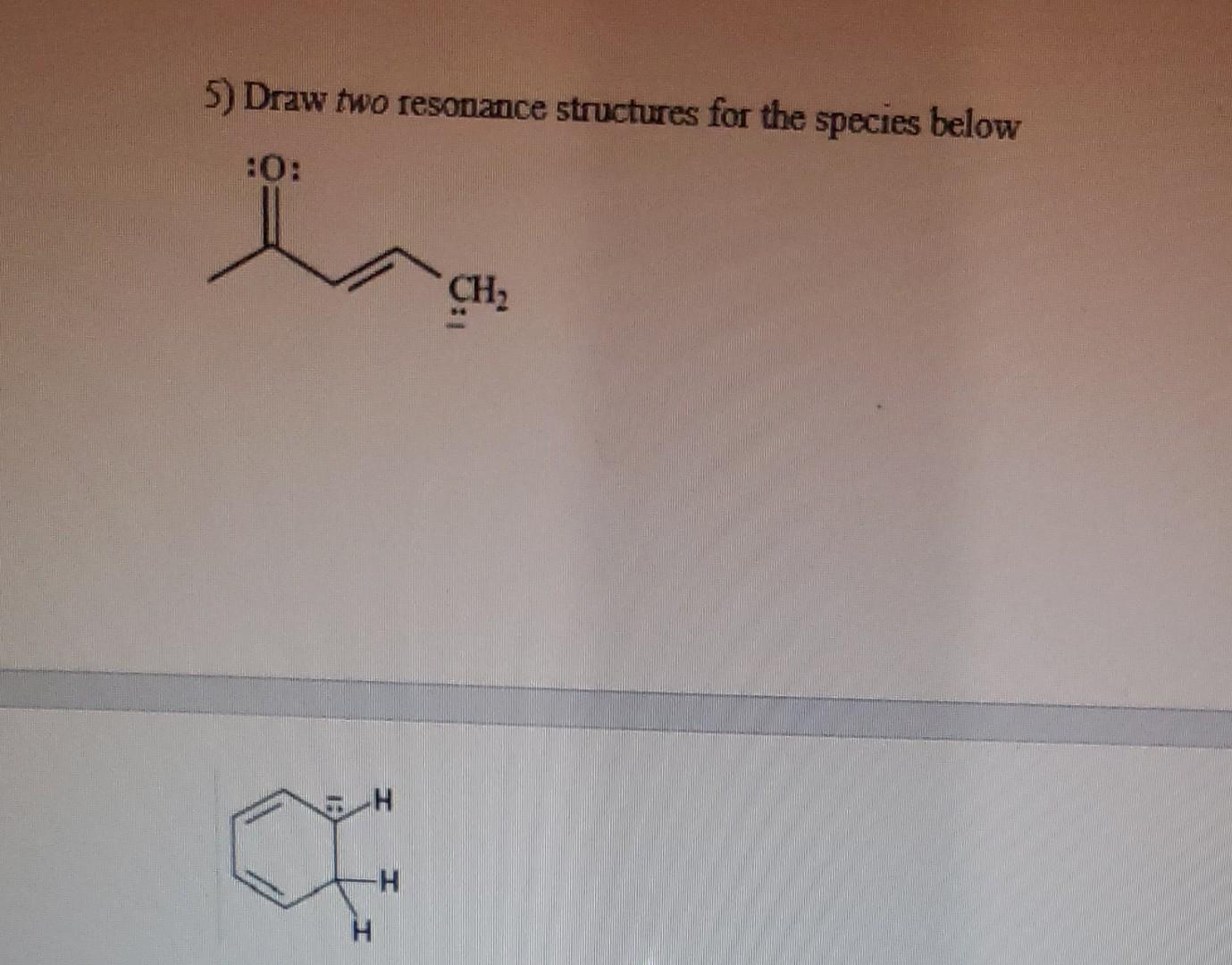
Solved 5 Draw Two Resonance Structures For The Species Che Question: 5. draw two resonance structures for the species below. 6. use the δ 18 convention and the crossed arrow ( ) to show the direction ofthe polarity of the c o bond in furan:. 5) draw two resonance structures for the species below your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.
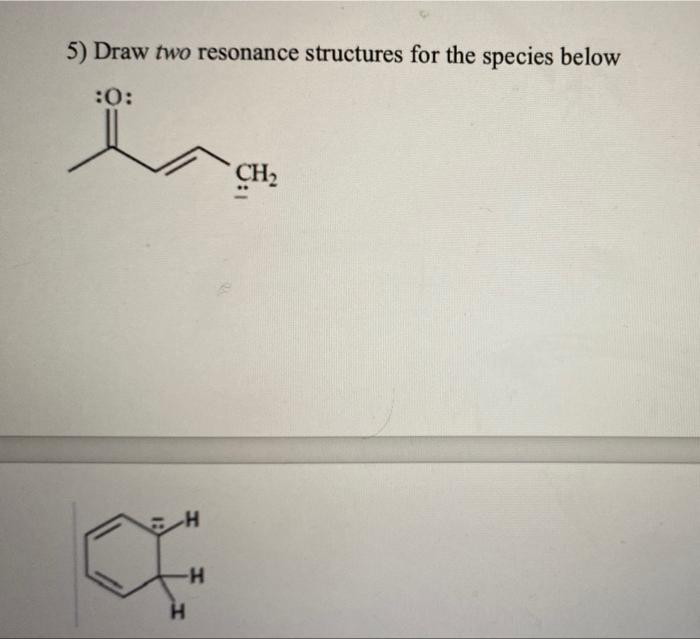
Solved 5 Draw Two Resonance Structures For The Species Che Question: 5. draw two resonance structures for the species below 6. show how to convert 1 butene to each of these compounds. use any reagents as necessary. c) butane d) 1,2 butanediol e) 1 butanol f) 2 butanol g) 1,2 dibromobutane. The nitrate (no − 3) ion. 1. count up the valence electrons: (1*5) (3*6) 1 (ion) = 24 electrons. 2. draw the bond connectivities: the three oxygens are drawn in the shape of a triangle with the nitrogen at the center of the triangle. 3. add octet electrons to the atoms bonded to the center atom: 4. Solution: the two resonance structures in this example are non equivalent, so one is more stable than the other.by applying the formal charge guideline, the “ “ formal charge is more preferable on oxygen, which is more electronegative than nitrogen, so the 2 nd structure is the more stable one with lower energy, and makes more contribution to the actual structure in this species. In summary, there are two must follow rules when drawing resonance structures: 1) do not exceed the octet on 2nd row elements. 2) do not break single bonds. keeping these in mind, go ahead and work on the following practice problems on drawing curved arrows, missing resonance forms, and determining the more stable resonance structure.

Solved 5 Draw Two Resonance Structures For The Species Che Solution: the two resonance structures in this example are non equivalent, so one is more stable than the other.by applying the formal charge guideline, the “ “ formal charge is more preferable on oxygen, which is more electronegative than nitrogen, so the 2 nd structure is the more stable one with lower energy, and makes more contribution to the actual structure in this species. In summary, there are two must follow rules when drawing resonance structures: 1) do not exceed the octet on 2nd row elements. 2) do not break single bonds. keeping these in mind, go ahead and work on the following practice problems on drawing curved arrows, missing resonance forms, and determining the more stable resonance structure. Lone pair of electrons on a carbon atom. a carbocation. a double bond between 2 atoms of different electronegativities. double bonds inside of a ring. when drawing a molecule, it is best represented in the most neutral charged state as possible, representing the most stable state of the molecule. when there is a molecule that has a charge, you. Video transcript. resonance theory is used to represent the different ways that the same molecule can distribute its electrons. so what that means is that even though the connectivity or how atoms are connected isn't going to change, the electrons between them can move sometimes. and that's what resonance theory is all about.

Solved 5 Draw Two Resonance Structures For The Species Che Lone pair of electrons on a carbon atom. a carbocation. a double bond between 2 atoms of different electronegativities. double bonds inside of a ring. when drawing a molecule, it is best represented in the most neutral charged state as possible, representing the most stable state of the molecule. when there is a molecule that has a charge, you. Video transcript. resonance theory is used to represent the different ways that the same molecule can distribute its electrons. so what that means is that even though the connectivity or how atoms are connected isn't going to change, the electrons between them can move sometimes. and that's what resonance theory is all about.

Solved 5 Draw Two Resonance Structures For The Species Che

Comments are closed.