Solved 1 Review Constants Periodic Table Part A Find A U о

Solved 1 Review Constants Periodic Table Part A Find Step 1. if a body starts moving in a circle then the force acting on it are centrifugal and centripetal acce in review | constants periodic table the figure (figure 1) shows partial motion diagram. part a choose the correct motion diagram completed by adding acceleration vectors. figure < 1 of 1 > top view of motion in a horizontal plane. Download article. 1. read the periodic table from top left to bottom right. the elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. the atomic number is how many protons the element’s atom possesses.
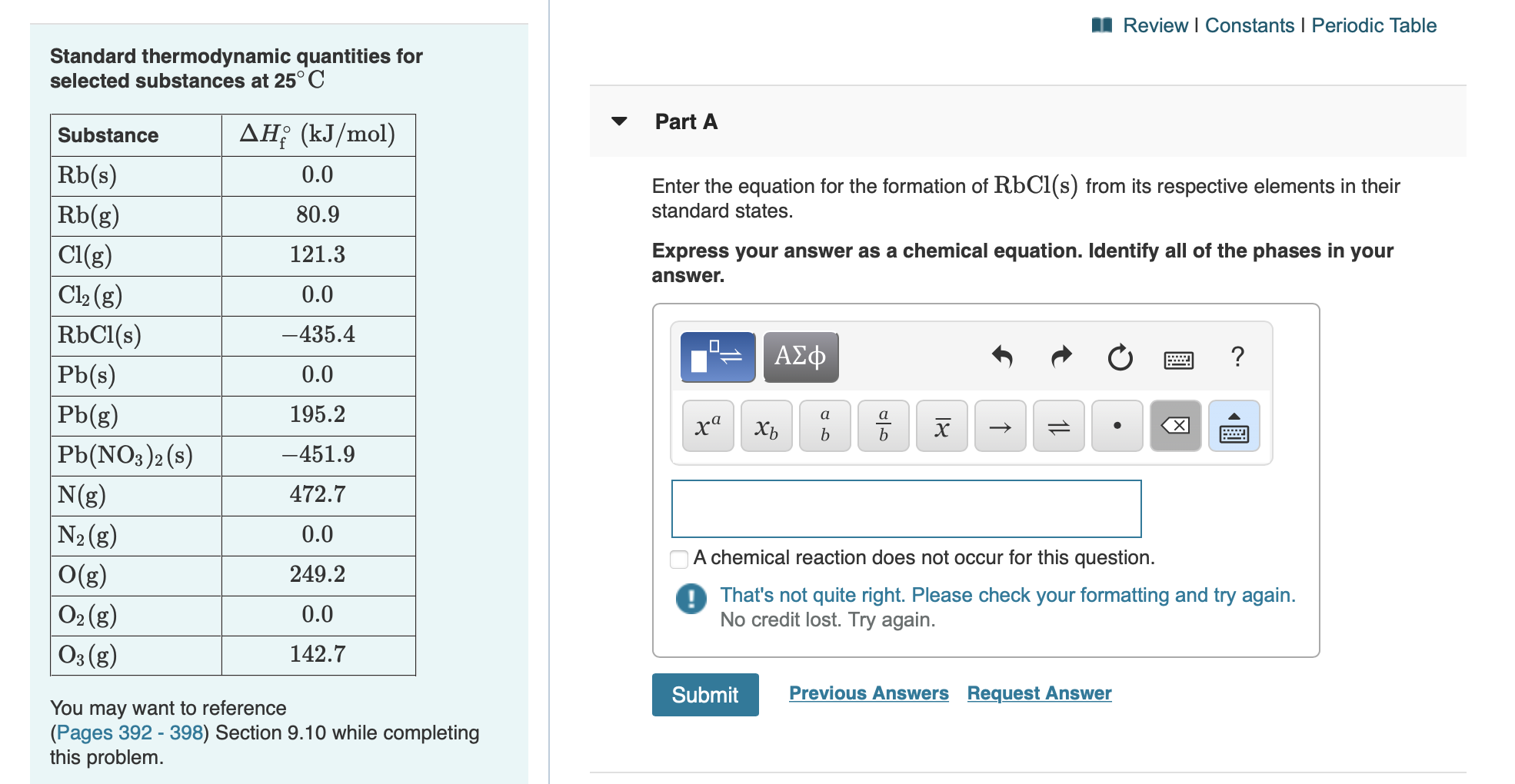
Solved 1 Review Constants Periodic Table Standard Chegg A more comprehensive description of the periodic table is found in chapter 7. figure 1.8.1 1.8. 1 the periodic table showing the elements in order of increasing z. the metals are on the bottom left in the periodic table, and the nonmetals are at the top right. the semimetals lie along a diagonal line separating the metals and nonmetals. Periodic table and constants. this is a copy of the periodic table as given in the "mastering essential pre university physical chemistry" book. the values on this table should be used where relevant to answer the problems from this book. Nacl (l) na (l) cl 2 (g) u n b a l a n c e d nacl (l) na (l) cl 2 (g) u n b a l a n c e d this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from 1 to 0 (it undergoes reduction ) and that for cl is increased from −1 to 0 (it undergoes oxidation ). Discover the interactive periodic table of elements with ptable, a web based tool that lets you explore the properties, trends, orbitals, isotopes, and compounds of each element. learn more about the elements with fully descriptive write ups and visualizations.

Solved U Review Constants 1 Periodic Table Part A Pr Nacl (l) na (l) cl 2 (g) u n b a l a n c e d nacl (l) na (l) cl 2 (g) u n b a l a n c e d this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from 1 to 0 (it undergoes reduction ) and that for cl is increased from −1 to 0 (it undergoes oxidation ). Discover the interactive periodic table of elements with ptable, a web based tool that lets you explore the properties, trends, orbitals, isotopes, and compounds of each element. learn more about the elements with fully descriptive write ups and visualizations. Question 9. use mendeleev's periodic table to predict the formula of: (a) hydrides of carbon and silicon. (b) oxides of potassium, aluminium and barium. answer. (a) carbon is in group 4. hydride of any element of group 4 is written as rh 4. hence, the hydride of carbon will have the formula ch4. Find selenium in the periodic table shown in figure 4.6.1 4.6. 1 and then classify the element according to its location. solution: the atomic number of selenium is 34, which places it in period 4 and group 16. in figure 4.6.1 4.6. 1, selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so.

Solved 1 Review Constants Periodic Table Part A Calculate Question 9. use mendeleev's periodic table to predict the formula of: (a) hydrides of carbon and silicon. (b) oxides of potassium, aluminium and barium. answer. (a) carbon is in group 4. hydride of any element of group 4 is written as rh 4. hence, the hydride of carbon will have the formula ch4. Find selenium in the periodic table shown in figure 4.6.1 4.6. 1 and then classify the element according to its location. solution: the atomic number of selenium is 34, which places it in period 4 and group 16. in figure 4.6.1 4.6. 1, selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so.

Comments are closed.