Solution Chemistry Acid Base Titration Studypool

Solution Acid Base Titration Method Studypool Vrogue Co It is also known as test solution. • indicator: auxiliary agents used to determine d point of titration. an indicator is weak acid or weak base, whose small amount is added to the analyte before starting titration and when titration take place then indicator changes its colour to show the completion of reaction. e.g. phenolphthalein changes. An acid base titration is a process which involves the gradual neutralization ofan acid or base until the equivalent point is reached, which is detected by the solution: analytical chemistry acid base titrations studypool.

Solution Chemistry Acid Base Titration Studypool Vrogue Co A titration is a common laboratory method used in analytical chemistry to determine the concentration of certain acids or bases during the production of certain biofuels, pharmaceutical drugs, and many other commercial products. in this assignment, you will perform an acid base titration of an unknown acid. instructions your task is to conduct an acid base titration. in this experiment, you. Indicator. references. acid base titrations are usually used to find the amount of a known acidic or basic substance through acid base reactions. the analyte (titrand) is the solution with an unknown molarity. the reagent (titrant) is the solution with a known molarity that will react with the analyte. Calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml. (b) 12.50 ml. (c) 25.00 ml. Types. an acid base titration is a fundamental process in analytical chemistry to determine the concentration of unknown acids or bases. it is based on the neutralization reaction, where an acid and a base react to form water and a salt. this method allows scientists and chemists to measure the amount of one substance precisely by reacting it.
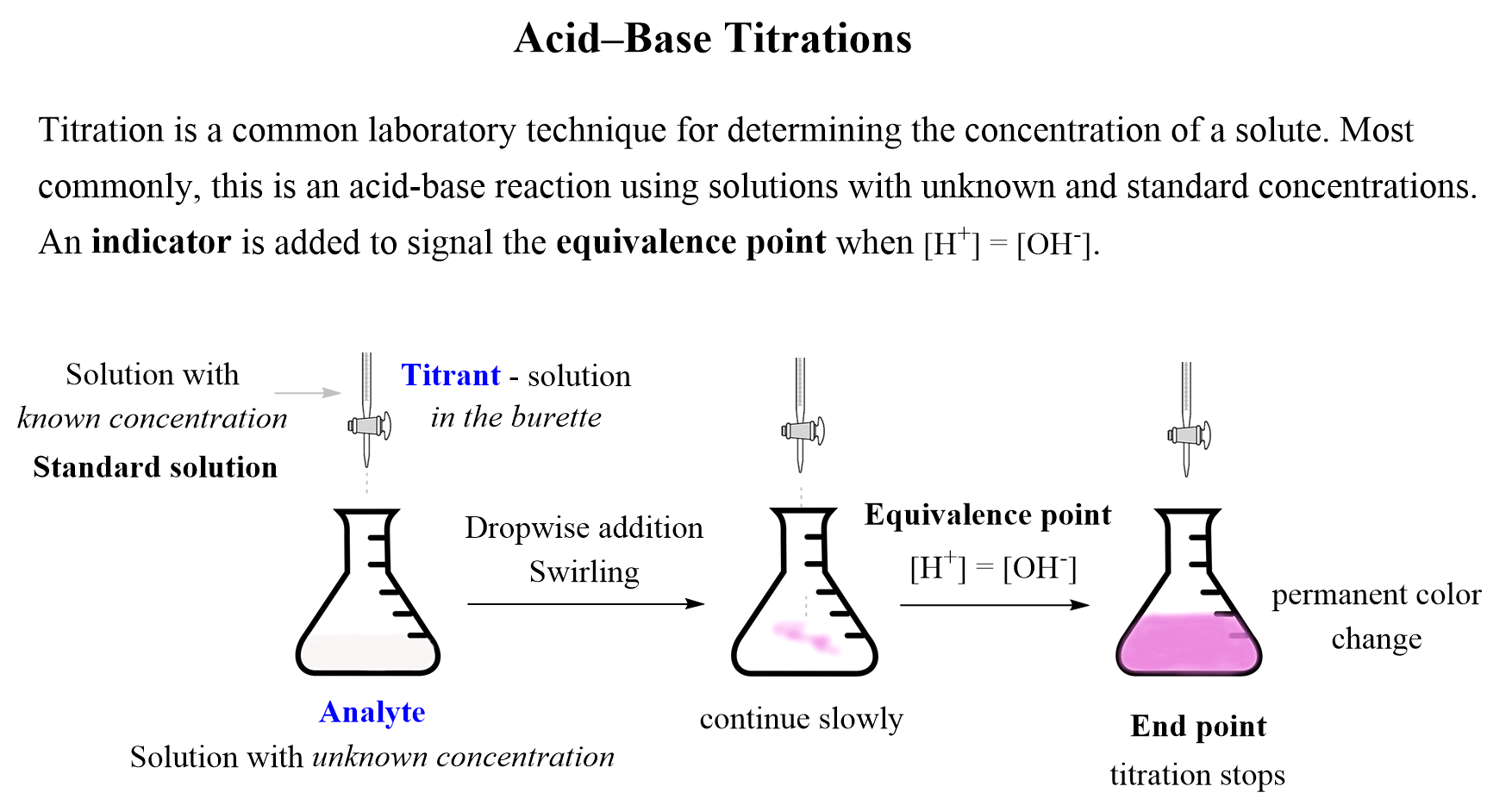
Strong Acidвђ Strong Base Titrations Chemistry Steps Calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml. (b) 12.50 ml. (c) 25.00 ml. Types. an acid base titration is a fundamental process in analytical chemistry to determine the concentration of unknown acids or bases. it is based on the neutralization reaction, where an acid and a base react to form water and a salt. this method allows scientists and chemists to measure the amount of one substance precisely by reacting it. Titration of a weak acid with a strong base. consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. the reaction can be represented as: ch 3 co 2 h oh − ch 3 co 2 − h 2 o. calculate the ph of the titration solution after the addition of the following volumes of naoh titrant: (a) 0.00 ml. Summary. plots of acid–base titrations generate titration curves that can be used to calculate the ph, the poh, the pka, and the pkb of the system. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration.
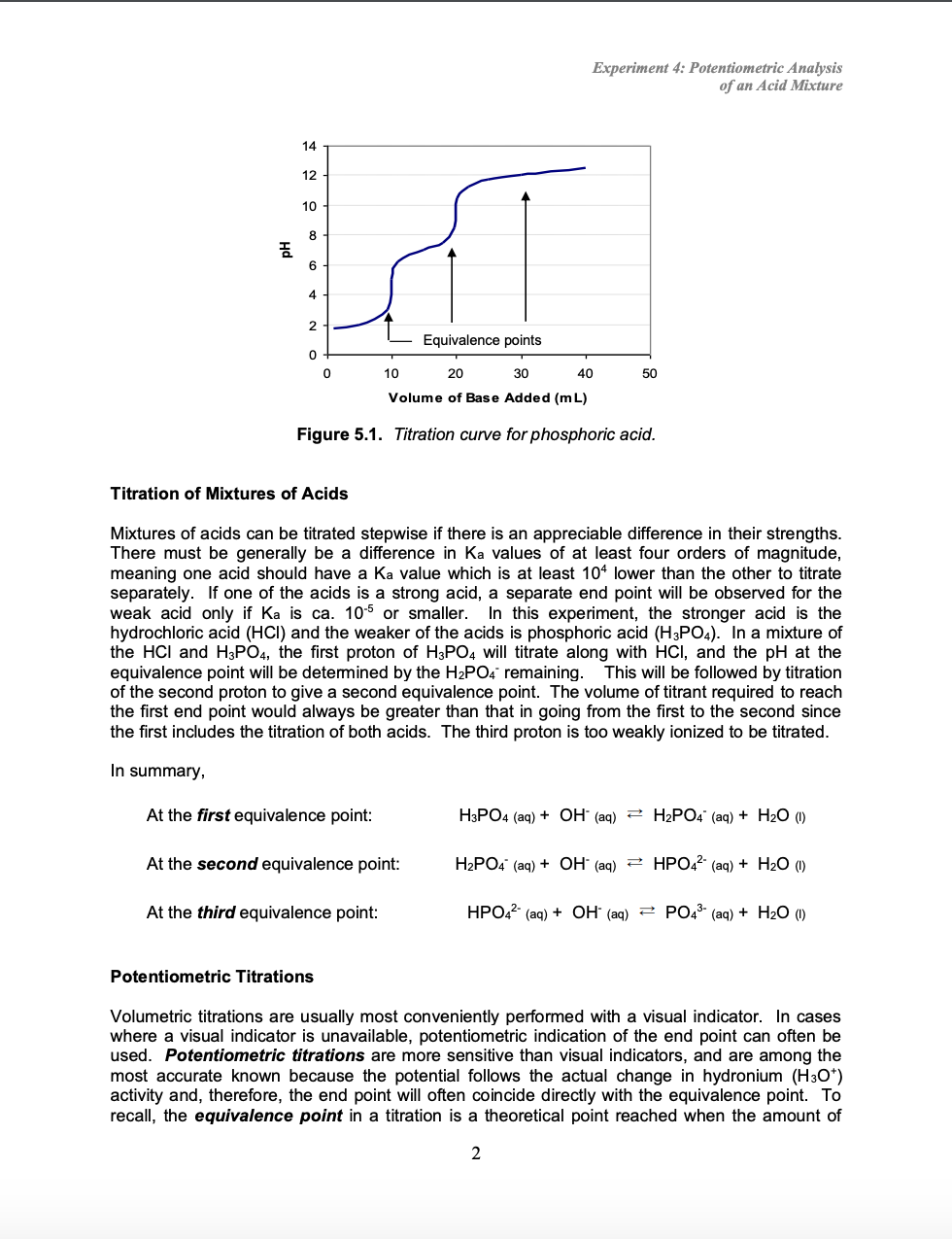
Solution Chemistry Acid Base Titration Studypool Vrogue Co Titration of a weak acid with a strong base. consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. the reaction can be represented as: ch 3 co 2 h oh − ch 3 co 2 − h 2 o. calculate the ph of the titration solution after the addition of the following volumes of naoh titrant: (a) 0.00 ml. Summary. plots of acid–base titrations generate titration curves that can be used to calculate the ph, the poh, the pka, and the pkb of the system. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration.

Comments are closed.