So2 Lewis Structure How To Draw The Lewis Structure For So2ођ
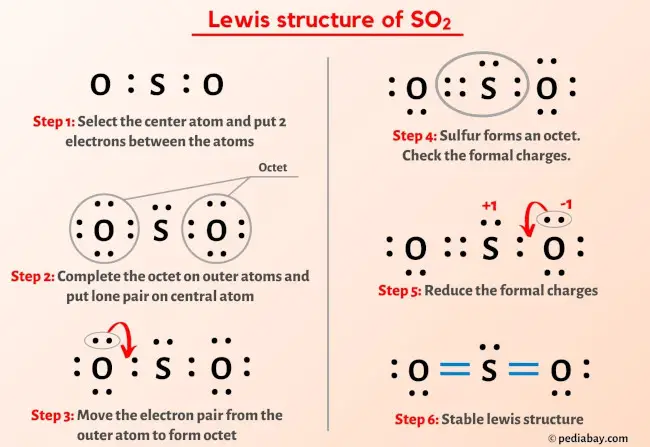
So2 Lewis Structure In 6 Steps With Images This chemistry video tutorial explains how to draw the lewis structure of so2 also known as sulfur dioxide. it discusses the molecular geometry, bond angle,. A step by step explanation of how to draw the so2 lewis structure (sulfur dioxide) note: from an experimental view (using x ray crystallography or someth.
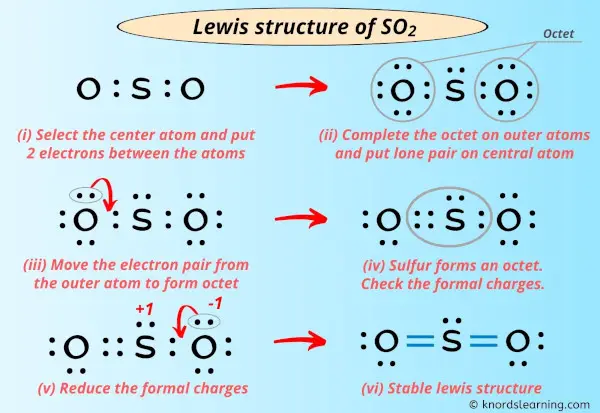
Lewis Structure Of So2 With 6 Simple Steps To Draw How to draw the lewis structure of so2 with explanationcheck me out: chemistnate. [voiceover] in the previous video, we looked at the dot structure for sulfur dioxide, and i drew out two resonance structures. so the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure for sulfur dioxide. To draw the so2 lewis structure, follow these simple steps: 1. determine the total valence electrons. start by counting the valence electrons of each atom in the molecule. in so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. therefore, the total valence electrons in so2. Step #1: calculate the total number of valence electrons. here, the given molecule is so2 (sulfur dioxide). in order to draw the lewis structure of so2, first of all you have to find the total number of valence electrons present in the so2 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom).

So2 Sulfur Dioxide Lewis Structure To draw the so2 lewis structure, follow these simple steps: 1. determine the total valence electrons. start by counting the valence electrons of each atom in the molecule. in so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. therefore, the total valence electrons in so2. Step #1: calculate the total number of valence electrons. here, the given molecule is so2 (sulfur dioxide). in order to draw the lewis structure of so2, first of all you have to find the total number of valence electrons present in the so2 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). 2. ) lewis structure, hybridization. sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. we will construct the lewis structure of so 2 molecule by following vsepr theory rules and considering stability of intermediate structures. after obtaining the lewis structure of so 2, we can determine the hybridization of atoms. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur (s). you might think you've got the correct lewis structure for so 2 at first. remember, sulfur is in period 3 and can hold more than 8 valence electrons. you'll want to calculate the formal charges on each atom to make sure you have the best lewis.

Lewis Structure Of Sulphur Dioxide So2 Youtube 2. ) lewis structure, hybridization. sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. we will construct the lewis structure of so 2 molecule by following vsepr theory rules and considering stability of intermediate structures. after obtaining the lewis structure of so 2, we can determine the hybridization of atoms. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur (s). you might think you've got the correct lewis structure for so 2 at first. remember, sulfur is in period 3 and can hold more than 8 valence electrons. you'll want to calculate the formal charges on each atom to make sure you have the best lewis.

Comments are closed.