Shapes Of Molecules
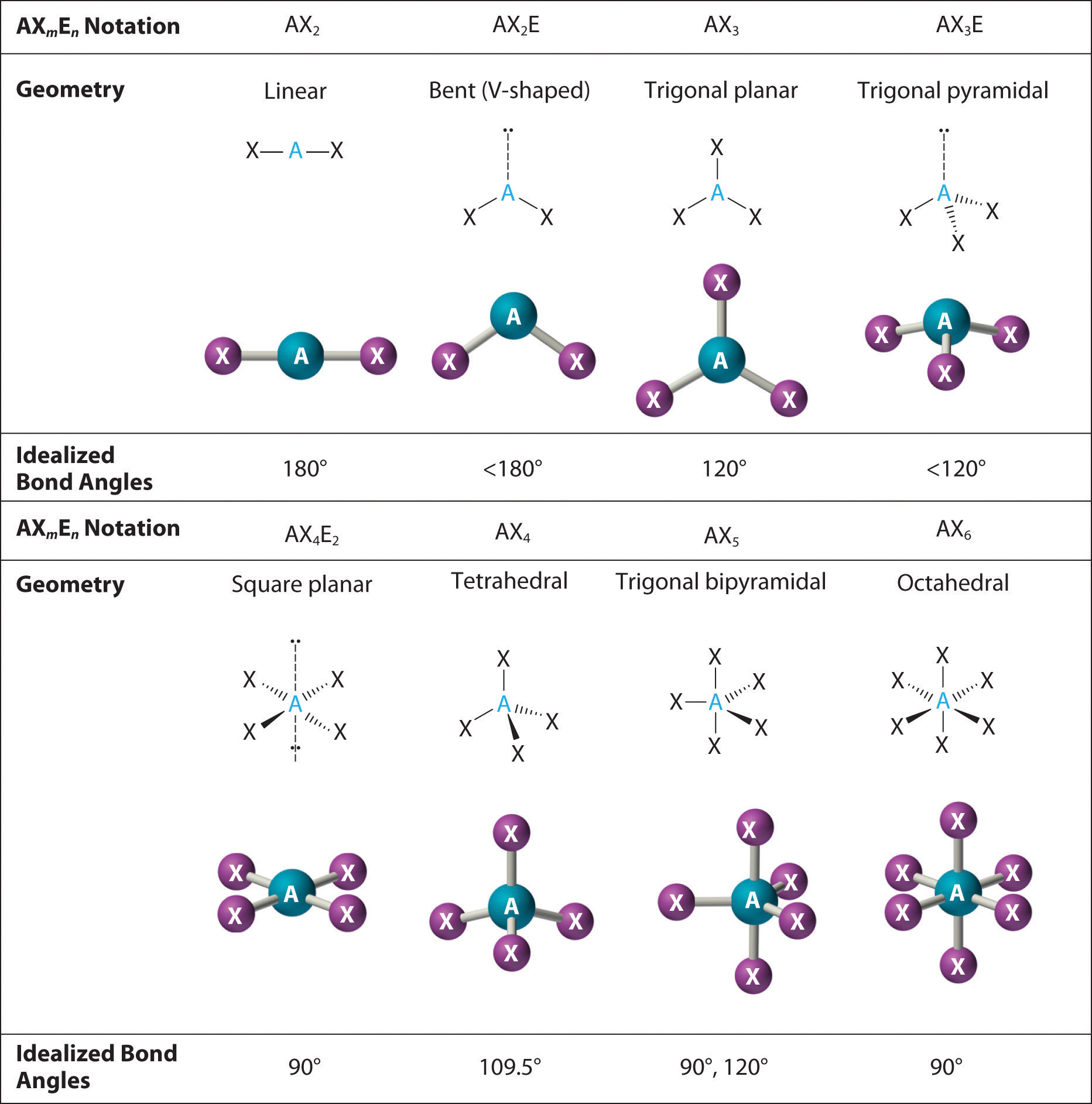
9 7 The Shapes Of Molecules Chemistry Libretexts 2. the carbon atom forms two double bonds. each double bond is a group, so there are two electron groups around the central atom. like beh 2, the arrangement that minimizes repulsions places the groups 180° apart. 3. once again, both groups around the central atom are bonding pairs (bp), so co 2 is designated as ax 2. Learn how to predict molecular shapes based on lewis diagrams and valence shell electron pair repulsion (vsepr) theory. see examples of different molecular geometries and their names, angles, and bond types.
Vsepr And The Shapes Of Molecules Chart Download Prin Vrogue Co Build molecules in 3d with different numbers of bonds and electron pairs. compare the model to real molecules and learn about vsepr, lone pairs and bonds. Learn how to determine the shape and bond angle of molecules using the vsepr theory and the molecular geometry chart. see examples of linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral molecules with and without lone pairs. Molecular geometry is the three dimensional arrangement of the atoms that constitute a molecule. it includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. molecular geometry influences several properties of a substance. Learn how to identify the molecular shape of a compound using the steric number and vsepr theory. find out how the shape affects the polarity, solubility, and bond angle of the molecule.

Shapes Of Simple Molecules вђ Chemistry Molecular geometry is the three dimensional arrangement of the atoms that constitute a molecule. it includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. molecular geometry influences several properties of a substance. Learn how to identify the molecular shape of a compound using the steric number and vsepr theory. find out how the shape affects the polarity, solubility, and bond angle of the molecule. Learn how to determine the shape of simple covalent molecules based on the number of bonding pairs and lone pairs of electrons. see examples, diagrams and explanations of linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral shapes. The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms. 6.4: molecular geometry is shared under a not declared license and was authored, remixed, and or curated by libretexts. molecules have shapes. there is an abundance of experimental evidence to that effect—from their.

Comments are closed.