Separation 1 What Processes Do You Know

Separation 1 What Processes Do You Know Youtube Evaporation. evaporation is a technique used to separate out homogeneous mixtures that contain one or more dissolved salts. the method drives off the liquid components from the solid components. the process typically involves heating the mixture until no more liquid remains. prior to using this method, the mixture should only contain one liquid. Mixtures can be separated using various separation methods such filtration,separating funnel,sublimation,simple distillation and paper chromatography. the methods stated above are all physical methods. there are also chemical methods, which are used by rearranging the particles so a certain substance no longer exists (chemical reaction).
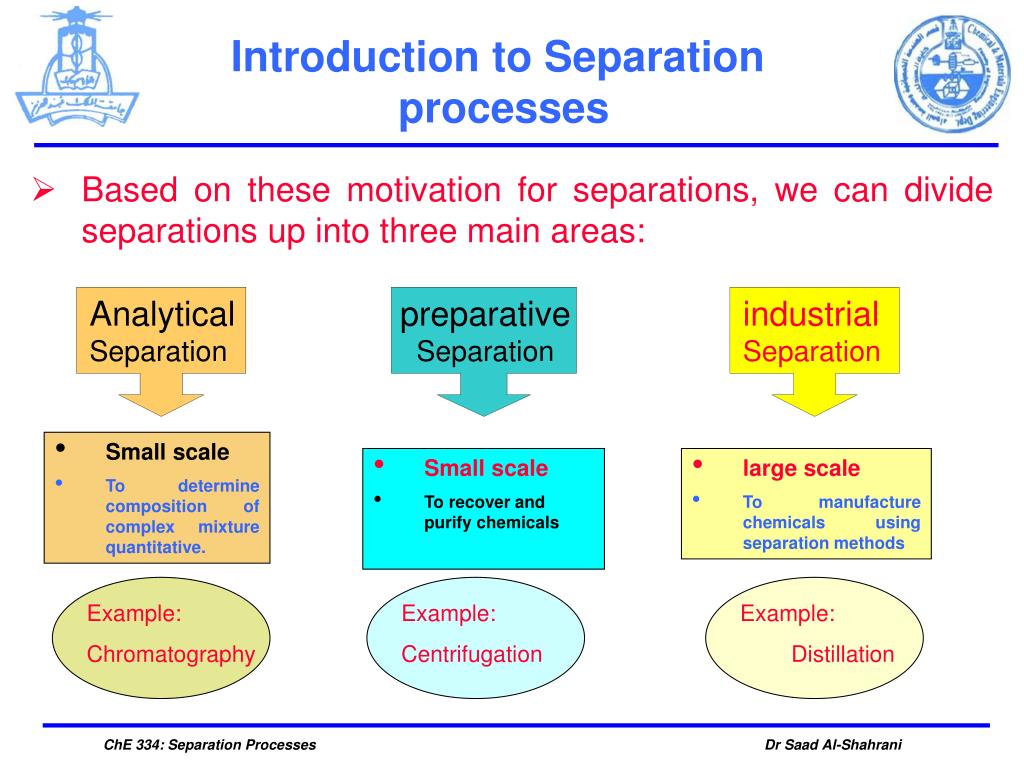
Ppt Che 334 Separation Processes Powerpoint Presentation Free V. t. e. a separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, [1] a scientific process of separating two or more substances in order to obtain purity. at least one product mixture from the separation is enriched in one or more of the source mixture's constituents. Preface. chemical engineering separations: a handbook for students is intended for use by undergraduate students who are taking a course in chemical engineering separations. the handbook assumes that students have taken one or two semesters of chemical engineering thermodynamics, one semester of heat and mass transfer, and one semester of. 7.6: classifying separation techniques. we can separate an analyte and an interferent if there is a significant difference in at least one of their chemical or physical properties. table 7.6.1 provides a partial list of separation techniques, organized by the chemical or physical property affecting the separation. As industries continually seek sustainable and cost effective separation processes, the significance of adsorption in achieving these goals cannot be overstated. 15. ion exchange: ion exchange is.

Methods Of Separation Learn Various Separation Techniques With Examples 7.6: classifying separation techniques. we can separate an analyte and an interferent if there is a significant difference in at least one of their chemical or physical properties. table 7.6.1 provides a partial list of separation techniques, organized by the chemical or physical property affecting the separation. As industries continually seek sustainable and cost effective separation processes, the significance of adsorption in achieving these goals cannot be overstated. 15. ion exchange: ion exchange is. Magnetic separation is the process of separating components of mixtures by using magnets to attract magnetic materials. the process that is used for magnetic separation detaches non magnetic material with those that are magnetic. 1. what method of separation would be most effective on the following mixtures: a) vinegar (a solution of acetic acid (liquid) in water) b) loose tea leaves in tea. c) copper sulfate (solid) in water. 2. identify what physical change occurs during the following separation processes.
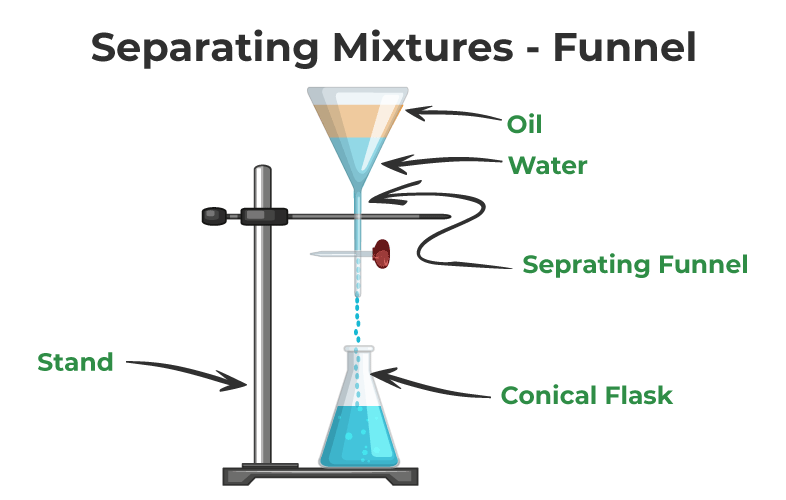
Methods Of Separation Various Separation Techniques Magnetic separation is the process of separating components of mixtures by using magnets to attract magnetic materials. the process that is used for magnetic separation detaches non magnetic material with those that are magnetic. 1. what method of separation would be most effective on the following mixtures: a) vinegar (a solution of acetic acid (liquid) in water) b) loose tea leaves in tea. c) copper sulfate (solid) in water. 2. identify what physical change occurs during the following separation processes.

Comments are closed.