Scale Up And Technology Transfer For Pharmaceuticals

Pdf Scale Up Technology Transfer As A Part Of Pharmaceutical Pharmaceutical quality systems “a common thread to tech transfer”. ich q10 “the change management system should provide management and documentation of adjustments made to the process during technology transfer activities.”. “aspects of management review should be performed to ensure the developed product and process can be. Technology scale up and transfer are common and crucial biodevelopment activities. for any biopharmaceutical manufacturer, commercial success depends on being able to increase drug substance production volume quickly and effectively and to move production freely without being locked into one provider. successful transfer is vital for product.
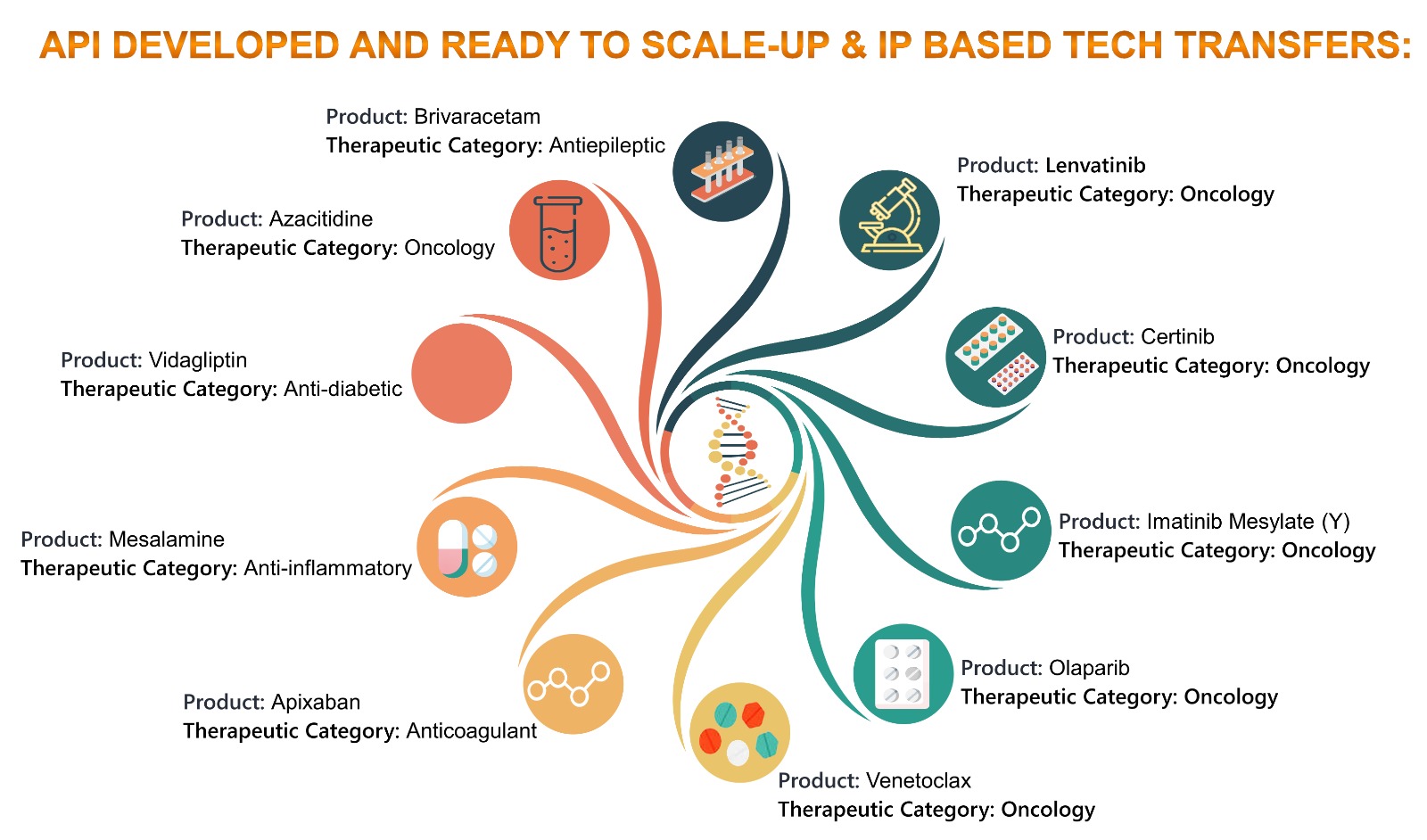
Scaleup Tech Transfer Myosynth Laboratories Pvt Ltd Conclusion. technology transfer is a very common and mature process in today’s pharmaceutical industry. however, it still poses many challenges for each transfer due to different product processes, equipment design and scale, regulatory needs, communication skills, and geographic locations. Pharmaceutical technology, pharmaceutical technology, november 2022, volume 46, issue 11 adherence to detail and thorough project management are required for successful tech transfer and scale up. a technology transfer is the moving of a product and process knowledge between development and manufacturing and within or between manufacturing. 1.1 technology transfer is a logical procedure involving the transfer of products, processes and knowledge, supported by relevant documentation and professional expertise. technology transfer may include development, manufacturing and testing sites. 1.2 the transfer of production and control procedures of pharmaceutical. This phase is referred to as stage 1b. stage 1b involves appropriate scaling, scale up down of the solid dose manufacturing unit operations to adapt to the requisite manufacturing capability while meeting the established cpps and cqas. product process knowledge management and scientific knowledge of product development and manufacturing science.

Comments are closed.