Redox Reactions Examples Types Applications Balancing
Redox Reactions Examples Types Applications Balancing Examples of redox reactions. a few examples of redox reactions, along with their oxidation and reduction half reactions, are provided in this subsection. example 1: reaction between hydrogen and fluorine. in the reaction between hydrogen and fluorine, the hydrogen is oxidized, whereas the fluorine is reduced. the reaction can be written as follows. Solution: (a) this is a decomposition reaction because one reactant is converted to two different products. – the oxidation number of n changes from 1 to 0, while that of o changes from 2 to 0. (b) this is a combination reaction (two reactants form a single product). – the oxidation number of li changes from 0 to 1 while that of n.

Redox Reactions Examples Types Applications Balancing Redox reaction is a class of reactions in which oxidation and reduction reactions occur simultaneously. in the reaction : 2 kclo 3 → 2 kcl 3 o 2. cl is reduced from 5 to 1 oxidation state and o is oxidized from 2 to 0 oxidation state. so, it is an example of a redox reaction. hence, the answer is the option (4). If there is no change in oxidation numbers, then the reaction is not a redox reaction. to illustrate the difference, here is an example of a redox reaction and a reaction that is not a redox reaction. redox reaction example: combustion of propane. the combustion of propane (c 3 h 8) in the presence of oxygen is a classic example of a redox. Solution. to balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and reduction, and balance them. step 1: split into two half reaction equations: oxidation and reduction. step 2: balance each of the half equations in this order:. Solution. step 1: separate the half reactions. by searching for the reduction potential, one can find two separate reactions: cu (aq) e − → cu(s) and. fe3 (aq) 3e − → fe(s) the copper reaction has a higher potential and thus is being reduced. iron is being oxidized so the half reaction should be flipped.

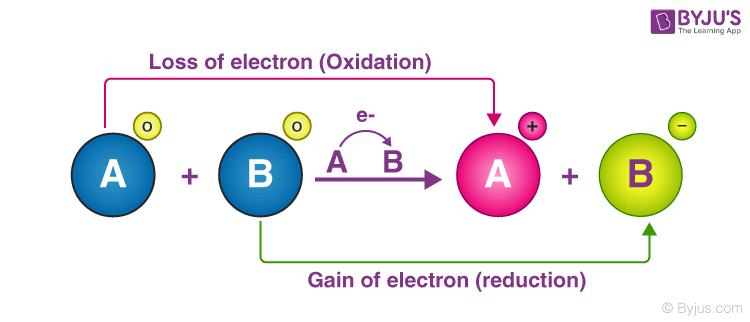
Redox Reactions Examples Types Applications Balancing Solution. to balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and reduction, and balance them. step 1: split into two half reaction equations: oxidation and reduction. step 2: balance each of the half equations in this order:. Solution. step 1: separate the half reactions. by searching for the reduction potential, one can find two separate reactions: cu (aq) e − → cu(s) and. fe3 (aq) 3e − → fe(s) the copper reaction has a higher potential and thus is being reduced. iron is being oxidized so the half reaction should be flipped. A redox reaction is a chemical reaction in which the atoms change their oxidation numbers. some atoms lose electrons and are oxidized – a process known as oxidation. on the other hand, some atoms gain electrons and are reduced – a process known as reduction. therefore, both reduction and oxidation take place simultaneously, hence the term. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. for example, in the redox reaction of na and cl 2: na cl2 → nacl. it should be immediately clear that the cl atoms are not balanced. we can fix this by putting the coefficient 2 in front of the product: na cl2 → 2nacl.
Redox Reactions Examples Types Applications Balancing A redox reaction is a chemical reaction in which the atoms change their oxidation numbers. some atoms lose electrons and are oxidized – a process known as oxidation. on the other hand, some atoms gain electrons and are reduced – a process known as reduction. therefore, both reduction and oxidation take place simultaneously, hence the term. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. for example, in the redox reaction of na and cl 2: na cl2 → nacl. it should be immediately clear that the cl atoms are not balanced. we can fix this by putting the coefficient 2 in front of the product: na cl2 → 2nacl.

Redox Oxidation Reduction Reaction Definition Examples

Comments are closed.