Protein Folding Gamma
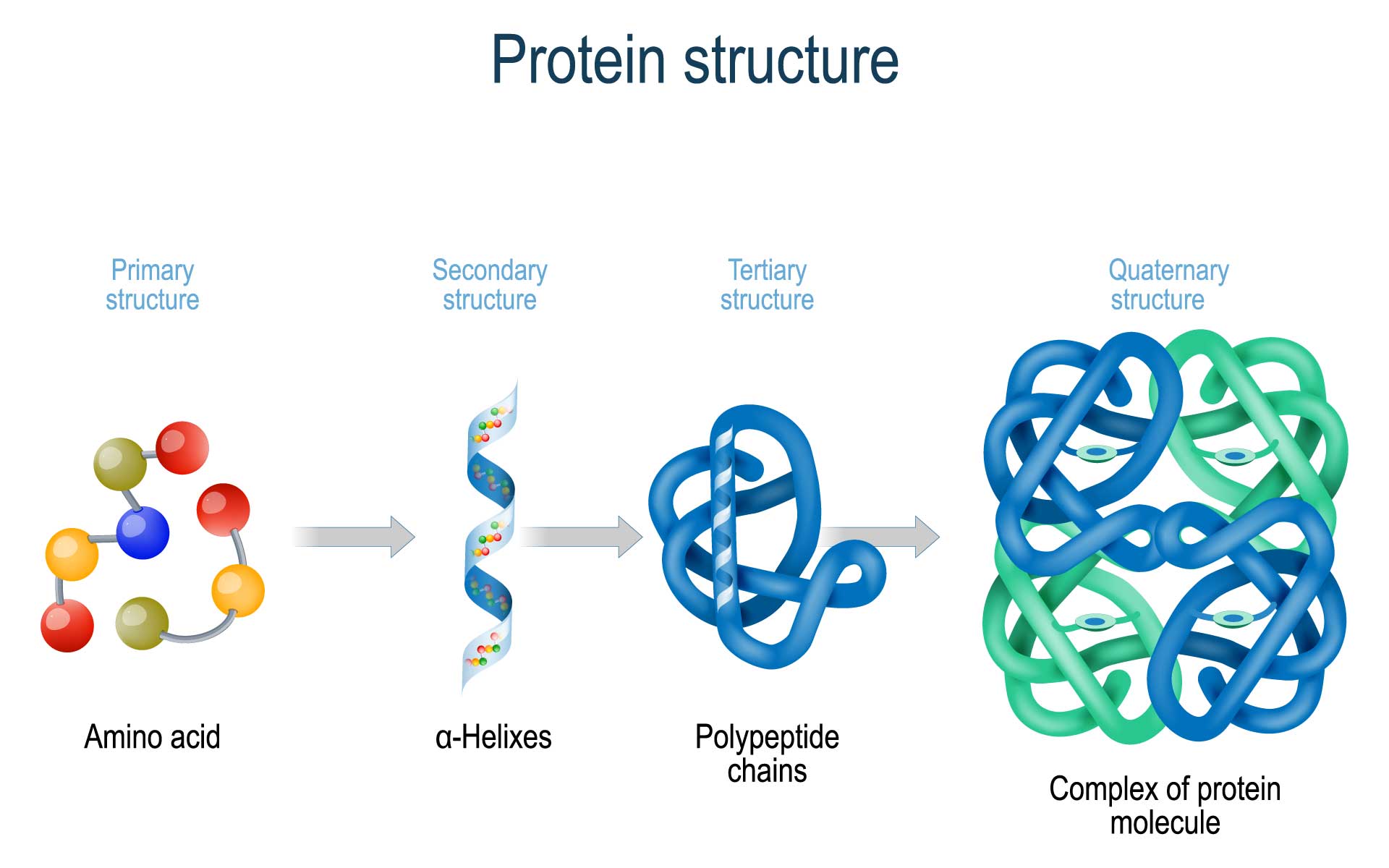
Protein Folding Gamma Our model successfully predicts protein folding processes consistent with experiments, without the limitations of protein size and shape. {\alpha,\beta,\gamma,\delta }\), should be multiplied. The correct folding is a key process for a protein to acquire its functional structure and conformation. prefoldin is a well known chaperone protein that regulates the correct folding of proteins. prefoldin plays a crucial role in the pathogenesis of common neurodegenerative diseases (alzheimer’s disease, parkinson’s disease, and huntington’s disease). the important role of prefoldin in.

Scheme 1 Schematic Protein Folding In The Presence Of Pmdp оі Fe 2 O 3 The mechanism of folding for this family of proteins depends on the formation of the β hairpin with the lower free energy at the transition state, making it possible to inter convert the folding pathway of proteins g and l by switching the intrinsic turn propensities of the two hairpins. 77 a redesigned protein g mutant was constructed by. These regions are known as random coils and are found in two locations in proteins: terminal arms both at the n terminus and the c terminus of the protein; loops loops are unstructured regions found between regular secondary structure elements. random coils can be 4 to 20 residues long, although most loops are not longer than 12 residues. Protein fold class. in molecular biology, protein fold classes are broad categories of protein tertiary structure topology. they describe groups of proteins that share similar amino acid and secondary structure proportions. each class contains multiple, independent protein superfamilies (i.e. are not necessarily evolutionarily related to one. Chapter 6 protein structure & folding 5 added to each side of the sheet. this can produce insoluble aggregates, or clumps of protein. in alzheimer’s disease a protein in the brain is cleaved, and one of the resulting fragments forms a highly extensive β sheet that aggregates to form plaques whose presence is correlated with tissue death.

The Protein Folding Problem 50 Years On Science Protein fold class. in molecular biology, protein fold classes are broad categories of protein tertiary structure topology. they describe groups of proteins that share similar amino acid and secondary structure proportions. each class contains multiple, independent protein superfamilies (i.e. are not necessarily evolutionarily related to one. Chapter 6 protein structure & folding 5 added to each side of the sheet. this can produce insoluble aggregates, or clumps of protein. in alzheimer’s disease a protein in the brain is cleaved, and one of the resulting fragments forms a highly extensive β sheet that aggregates to form plaques whose presence is correlated with tissue death. Folding rate as a function of the geometry and topology of the native state for a mixed (two state and multi state) set of proteins. figure 4 shows the logarithm of the experimental folding rate. Protein folding. proteins are folded and held together by several forms of molecular interactions. the molecular interactions include the thermodynamic stability of the complex, the hydrophobic interactions and the disulfide bonds formed in the proteins. the figure below (figure 2 2) is an example of protein folding.
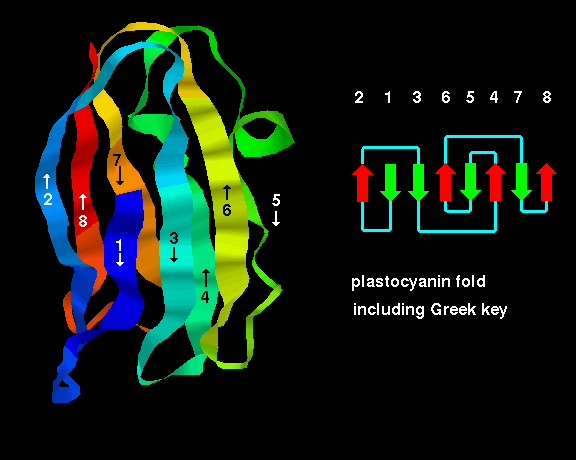
Protein Folds All Beta Folding rate as a function of the geometry and topology of the native state for a mixed (two state and multi state) set of proteins. figure 4 shows the logarithm of the experimental folding rate. Protein folding. proteins are folded and held together by several forms of molecular interactions. the molecular interactions include the thermodynamic stability of the complex, the hydrophobic interactions and the disulfide bonds formed in the proteins. the figure below (figure 2 2) is an example of protein folding.

Comments are closed.