Primary Cutaneous T Cell Lymphoma Alcanza Trial

Primary Cutaneous T Cell Lymphoma Alcanza Trial The primary analysis of the phase 3 alcanza trial showed significantly improved objective responses lasting ≥4 months (orr4; primary endpoint) and progression free survival (pfs) with brentuximab vedotin vs physician's choice (methotrexate or bexarotene) in cd30 expressing mycosis fungoides (mf) or primary cutaneous anaplastic large cell. Introduction. cutaneous t cell lymphomas (ctcls) represent a heterogeneous group of t cell lymphomas, primarily involving the skin. mycosis fungoides (mf) and primary cutaneous anaplastic large cell lymphoma (c alcl) are 2 of the most common subtypes of ctcl. 1,2 despite being distinct malignancies, mf and c alcl share some clinical and pathological characteristics: they both express cluster.

Tsebt Vorinostat For Cutaneous T Cell Lymphoma Clinical Trial 2024 Cutaneous t cell lymphomas (ctcls) are a group of rare non hodgkin lymphomas that present primarily in the skin. 1 in the united states, the estimated incidence of ctcl is 0.69 cases per 100,000 person years; it more commonly occurs in men and is typically diagnosed in adults between 50 and 70 years of age. 2 clinical presentation is highly variable, making definitive diagnosis challenging. 1. These real world outcomes are consistent with alcanza results, demonstrating favorable outcomes with bv vs. ost in patients with ctcl previously treated with ≥1 systemic therapy. real world treatment patterns and clinical outcomes with brentuximab vedotin or other standard therapies in patients with previously treated cutaneous t cell. Methods: in this international, open label, randomised, phase 3, multicentre trial, we enrolled adult patients with cd30 positive mycosis fungoides or primary cutaneous anaplastic large cell lymphoma who had been previously treated. patients were enrolled across 52 centres in 13 countries. The clinical benefit reported in this article builds on the previous positive reports of brentuximab vedotin for the treatment of cd30 positive cutaneous t cell lymphoma in the relapsed or refractory setting. 17,18 this study represents the first demonstration of benefit in a randomised phase 3 trial with a novel systemic drug versus an active.

Brentuximab Vedotin Or Physician S Choice In Cd30 Positive Cutaneous T Methods: in this international, open label, randomised, phase 3, multicentre trial, we enrolled adult patients with cd30 positive mycosis fungoides or primary cutaneous anaplastic large cell lymphoma who had been previously treated. patients were enrolled across 52 centres in 13 countries. The clinical benefit reported in this article builds on the previous positive reports of brentuximab vedotin for the treatment of cd30 positive cutaneous t cell lymphoma in the relapsed or refractory setting. 17,18 this study represents the first demonstration of benefit in a randomised phase 3 trial with a novel systemic drug versus an active. In cutaneous t cell lymphoma. we did not identify any phase 3 studies of brentuximab vedotin for cutaneous t cell lymphoma. added value of this study. this is the first randomised study of a new systemic drug against . standard therapy and the largest reported phase 3 trial in patients with cutaneous t cell lymphoma, and unlike many. The open label phase iii alcanza trial randomized 131 patients with cd30 expressing ctcl, including patients with primary cutaneous anaplastic large cell lymphoma (pcalcl) or mycosis fungoides (mf).
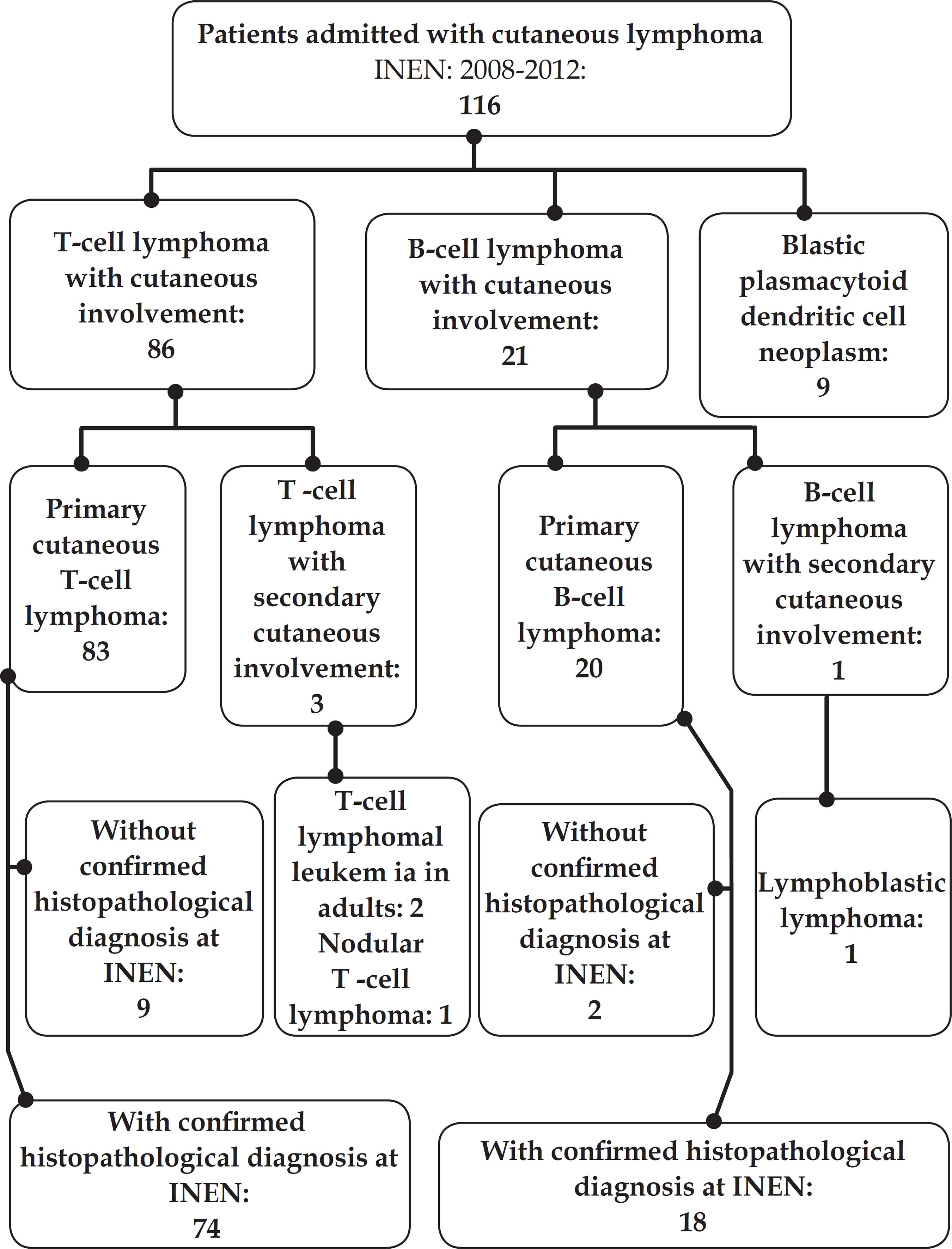
Scielo Brasil Primary Cutaneous T Cell Lymphoma Experience From In cutaneous t cell lymphoma. we did not identify any phase 3 studies of brentuximab vedotin for cutaneous t cell lymphoma. added value of this study. this is the first randomised study of a new systemic drug against . standard therapy and the largest reported phase 3 trial in patients with cutaneous t cell lymphoma, and unlike many. The open label phase iii alcanza trial randomized 131 patients with cd30 expressing ctcl, including patients with primary cutaneous anaplastic large cell lymphoma (pcalcl) or mycosis fungoides (mf).

Comments are closed.