Preparing A Standard Solution Chemistry
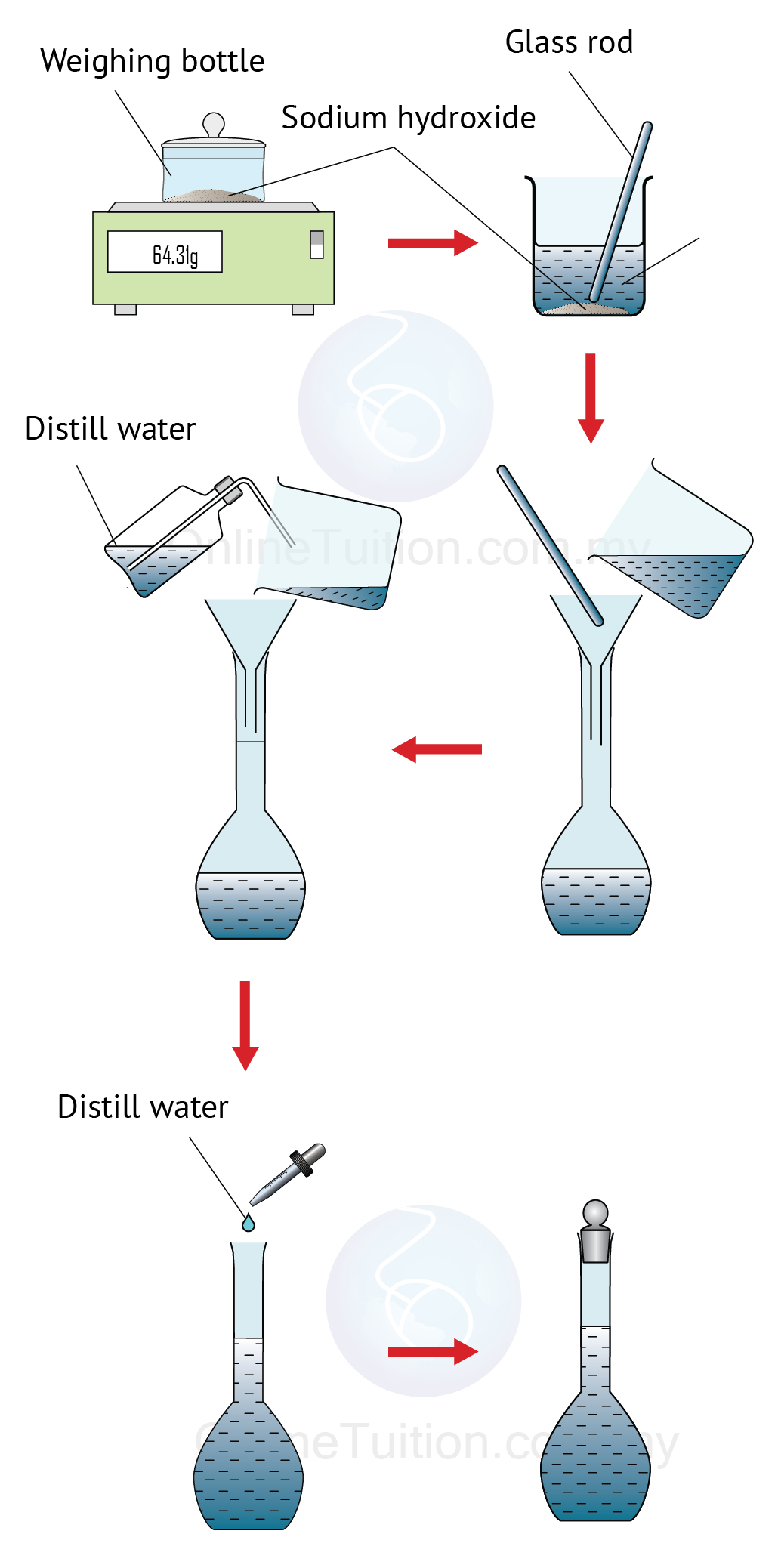
Preparing Standard Solutions Spm Chemistry D) the techniques and procedures used when preparing a standard solution of required concentration and carrying out acid–base titrations; edexcel chemistry. core practicals. 2. prepare a standard solution from a solid acid and use it to find the concentration of a solution of sodium hydroxide; scotland. higher. sqa chemistry. 3. chemistry in. Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions, such as strong acids. the procedure for preparing a solution of known concentration from a stock solution is shown in figure 12.1.3. it requires calculating the number of moles of solute desired in the final volume of the more dilute.
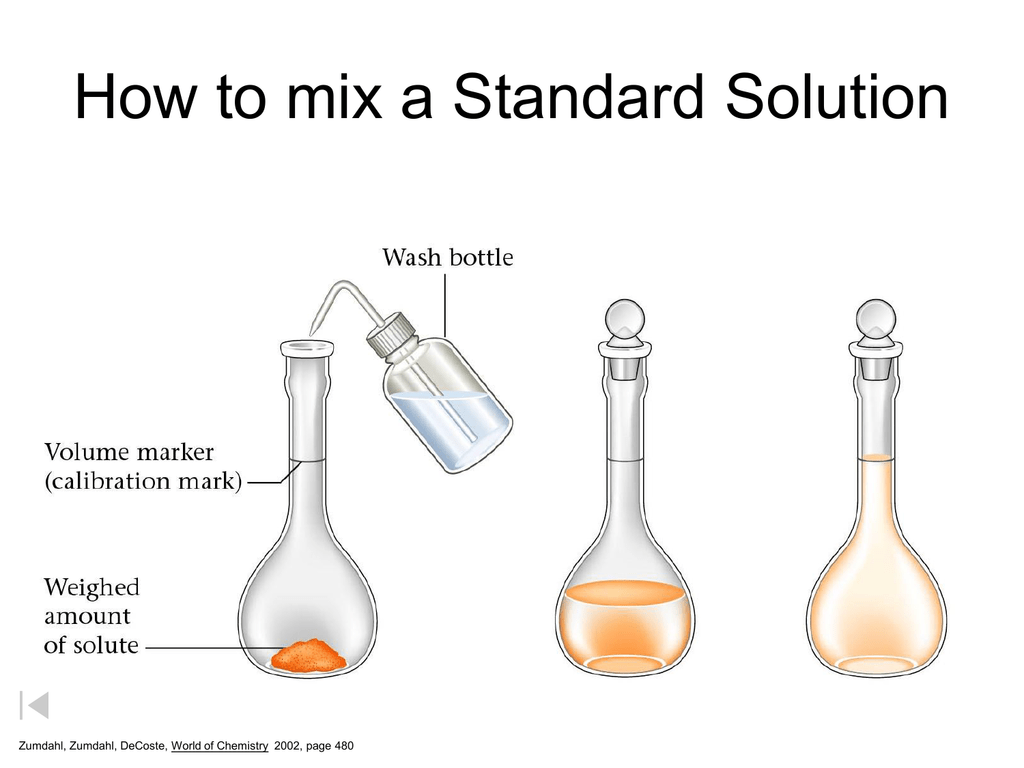
How To Mix A Standard Solution Watch how to prepare a standard solution.at the royal society of chemistry we provide education resources via our website learn chemistry to enhance teaching. Method #2: standard solution preparation by weighing. another way of preparing a standard solution is to follow the weighing method. typically used to prepare basic standard solutions, the weighing method requires a pure substance that can be dissolved in a solvent such as water. process of the weighing method. Step by step procedure for preparing a standard solution. 1. define the desired concentration and volume : before you start, determine the concentration and volume of the solution. this will dictate the amount of solute (solid or liquid) required. concentration is often expressed in molarity (m), where 1 m corresponds to one mole of solute per. In chemistry, a standard solution is one with a known concentration of a certain molecule or analyte. when preparing standard solutions, you might need to dissolve a primary standard in a.

How To Make A Standard Solution вђ Hsc Chemistry вђ Science Ready Step by step procedure for preparing a standard solution. 1. define the desired concentration and volume : before you start, determine the concentration and volume of the solution. this will dictate the amount of solute (solid or liquid) required. concentration is often expressed in molarity (m), where 1 m corresponds to one mole of solute per. In chemistry, a standard solution is one with a known concentration of a certain molecule or analyte. when preparing standard solutions, you might need to dissolve a primary standard in a. Standard solution. in analytical chemistry, a standard solution (titrant or titrator) is a solution containing an accurately known concentration. standard solutions are generally prepared by dissolving a solute of known mass into a solvent to a precise volume, or by diluting a solution of known concentration with more solvent. [1]. Solutions are often prepared by diluting a more concentrated stock solution. a known volume of the stock solution is transferred to a new container and brought to a new volume. since the total amount of solute is the same before and after dilution, we know that. co ×vo = cd ×vd (11.4.1) (11.4.1) c o × v o = c d × v d.

Comments are closed.