Preparation Of Soap Chemistry

Science Fair Experiments Soaps And Detergents For Std 8 To 10 Science The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of beef or mutton fat). making soap was a long and arduous process. first, the fat had to be rendered (melted and filtered). then, potash solution was added. since water and oil do not mix, this mixture had to be continuously stirred and heated. Preparation of soap by walter scharf and charles malerich natural sciences chemistry baruch college new york, ny 10010 introduction soap, from a chemical standpoint, is a salt (or a mixture of salts) of fatty acids. as with all salts, soap contains a positive ion, usually na or k , and a negative ion, usually the anions of.

Cbse Class 10 Science Lab Manual Soap Preparation A Plus Topper Step 1 measure the main ingredients. start by carefully measuring your chosen ingredients. many recipes use 40% lye (sodium hydroxide) concentration by volume dissolved in water, although feel free to experiment. the proportion of oil with the lye solution may vary depending on the type of oil you’re using. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. the water layer is drawn off the top of the mixture and the glycerol is recovered using vacuum. the crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide, and glycerol. these impurities are removed by boiling. Making soap – the saponification reaction. soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). the chemical structures of fats and oils generally look like this: the left hand side (purple) is always the same – it’s based on a glycerin (aka glycerol) molecule. Experiment 13 – preparation of soap. soaps are carboxylate salts with very long hydrocarbon chains. soap can be made from the base hydrolysis of a fat or an oil. this hydrolysis is called saponification, and the reaction has been known for centuries. traditionally, soaps were made from animal fat and lye (naoh).
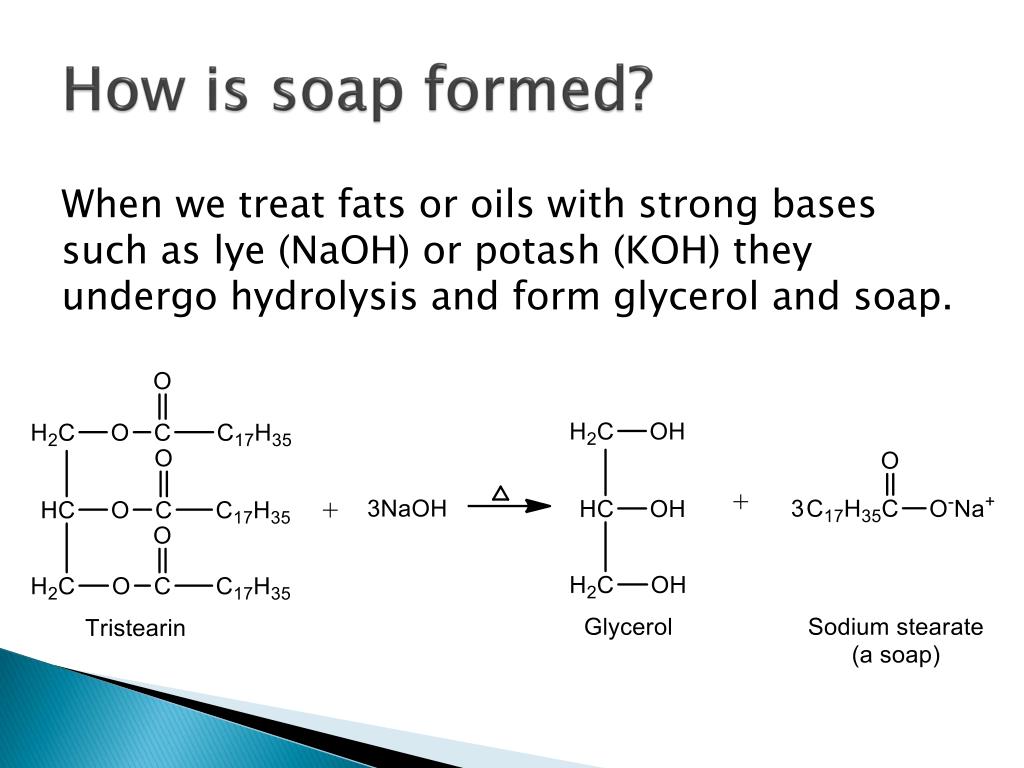
Ppt Preparation And Properties Of A Soap Powerpoint Presentation Making soap – the saponification reaction. soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). the chemical structures of fats and oils generally look like this: the left hand side (purple) is always the same – it’s based on a glycerin (aka glycerol) molecule. Experiment 13 – preparation of soap. soaps are carboxylate salts with very long hydrocarbon chains. soap can be made from the base hydrolysis of a fat or an oil. this hydrolysis is called saponification, and the reaction has been known for centuries. traditionally, soaps were made from animal fat and lye (naoh). William reusch, professor emeritus (michigan state u.), virtual textbook of organic chemistry. soaps and detergents. soap is manufactured by the base catalyzed hydrolysis (saponification) of animal fat. before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood …. Experiment 718: making soap saponification . section 1: purpose and summary . make lye soap from sodium hydroxide and olive oil via the saponification reaction. soapmaking is an ancient technique which includes the mixing of water, a solution formed from burned wood ashes and fats and or oils.
Physics Learn To Prepare Soap By Cold Process Science Practical Gseb William reusch, professor emeritus (michigan state u.), virtual textbook of organic chemistry. soaps and detergents. soap is manufactured by the base catalyzed hydrolysis (saponification) of animal fat. before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood …. Experiment 718: making soap saponification . section 1: purpose and summary . make lye soap from sodium hydroxide and olive oil via the saponification reaction. soapmaking is an ancient technique which includes the mixing of water, a solution formed from burned wood ashes and fats and or oils.
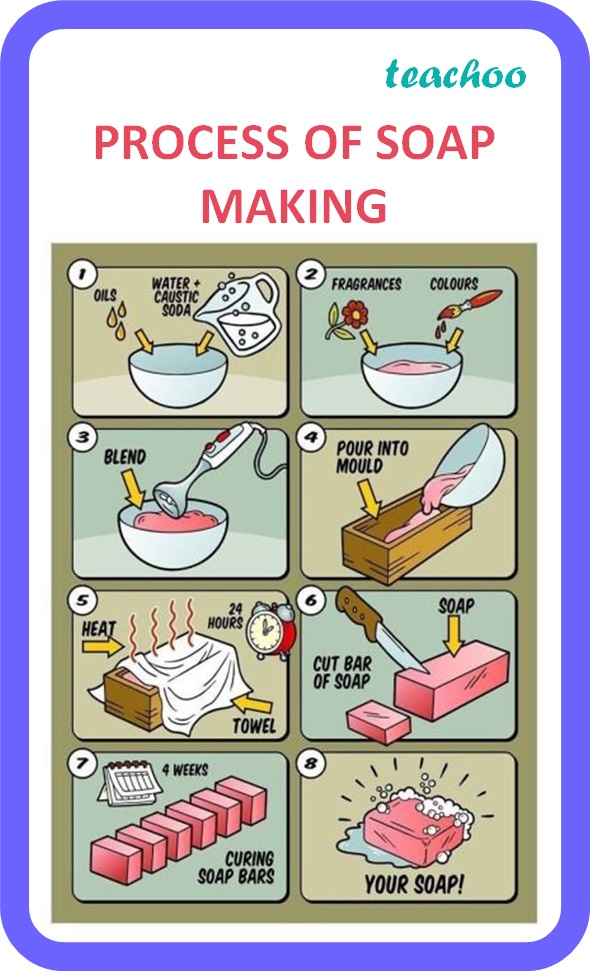
Preparation Of Soap In Chemistry Project

Comments are closed.