Preparation Of Benzoic Acid From The Grignard Reaction
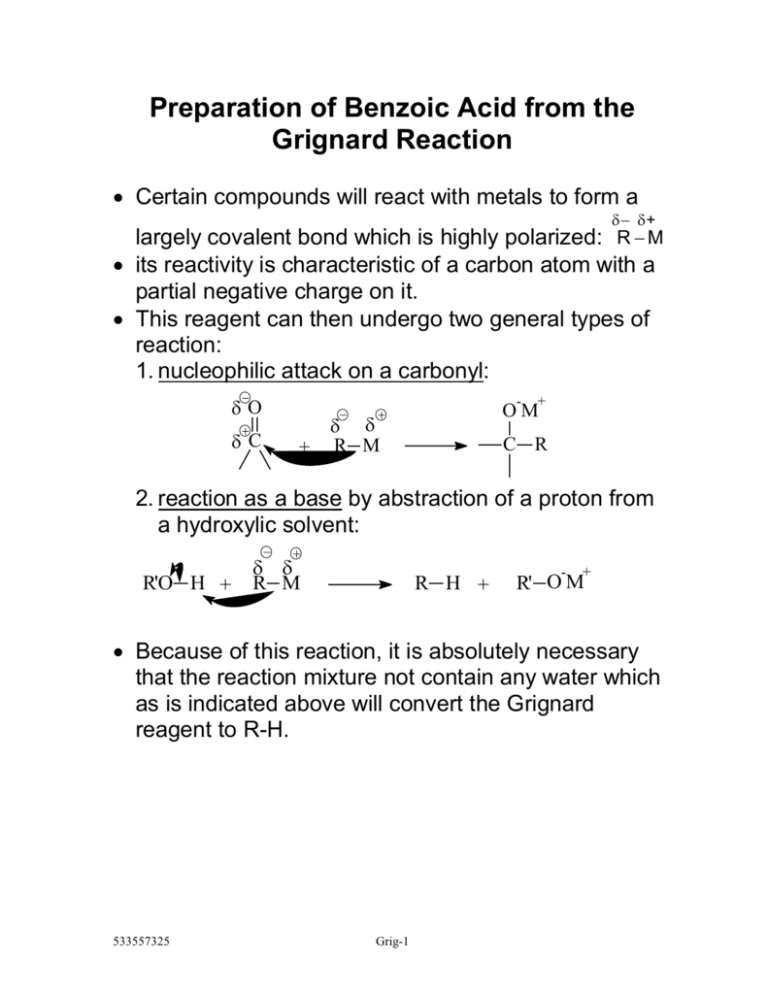
Preparation Of Benzoic Acid From The Grignard Reaction The reaction proceeds through the formation of an intermediate carboxylate salt, which is then protonated to yield benzoic acid. in conclusion, the synthesis of benzoic acid from a grignard reagent is a useful method for the preparation of carboxylic acids. the reaction is also highly selective, with no other major products formed and the yield. The grignard reaction: a microscale preparation of benzoic acid introduction your laboratory skills have grown considerably since the first of the semester, and you are ready for the challenge of a famous reaction one marked by unusual materials and striking chemical and physical changes. try, especially, to understand exactly which.

For The Experiment Preparation Of Benzoic Acid A Grignard Reaction Chemistry document from university of ottawa, 8 pages, task 3 introduction and reaction mechanism this lab focuses on the preparation of benzoic acid using a grignard reagent. grignard reagents are organometallic, meaning that they are composed of an organic component and a metal. grignards have the general s. Preparation of grignard reagent. obtain 3 ml of anhydrous diethyl ether from your gsi in one of the oven dried dram vials. weigh magnesium powder (50 mg, 2 mmol) and add it to your reaction vessel. using a 1.0 ml syringe inserted through the septum add 0.5 ml of anhydrous diethyl ether to the reaction vessel. in a separate oven dried vial, add. C) when the solid carbon dioxide has all gone, slowly add a mixture of water (7 ml), ice (7 g) and concentrated hydrochloric acid (1.0 ml). mix thoroughly. 3. isolating the benzoic acid. add the mixture to a separating funnel. extract the aqueous phase with two portions of ether (7 ml each). ie. keep the top layers. In this reaction, the grignard reagent (an organomagnesium compound), phenylmagnesium bromide is prepared by reaction of bromobenzene with magnesium metal in diethyl ether (the solvent). the grignard reagent will then be converted to benzoic acid via the reaction of the grignard reagent with excess dry ice (solid co2) followed by a dilute.

Comments are closed.