Ppt The Transition Metals Powerpoint Presentation Free Download Id

Ppt Transition Metals Powerpoint Presentation Free Downloadођ Presentation transcript. transition metals the d block elements. properties of transition metals • have a wide range of oxidation states (different positively charged ions formed) • malleable and very hard metals • high melting and boiling points • high electrical conductivity • low ionization energies • located in the d block. Transition metals. this document provides information about transition metals and their coordination compounds. it discusses the key characteristics of transition metals, including having multiple oxidation states and forming colored compounds. transition metals form coordination complexes by acting as lewis acids and coordinating with ligands.
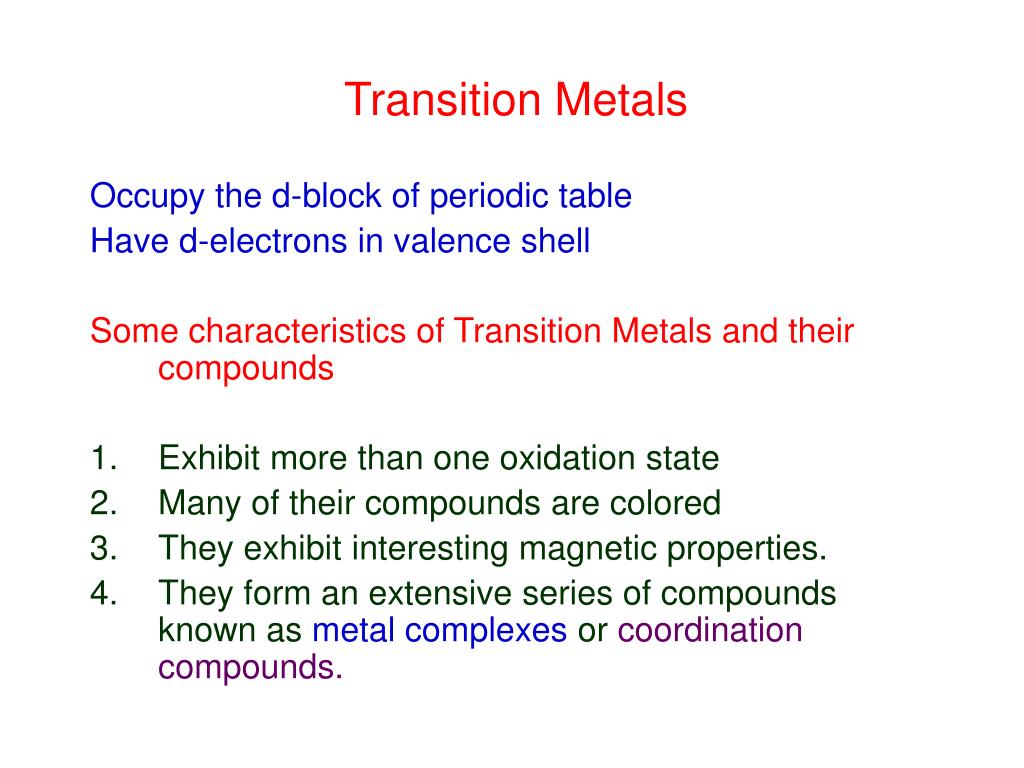
Ppt Transition Metals Powerpoint Presentation Free Downloadођ The three most commonly known transition elements are iron or steel, copper and zinc. iron or steel copper zinc electrical and plumbing work general engineering metal galvanising steel to protect it uses. pair the metal up with its uses copper iron or steel zinc. pair the metal catalyst with the substance. fe ni ti v. 14. metallic character all transition elements are metallic in nature metallic & covalant bonding valence s electron – metallic bond (n 1) d electron covalent bond lustre & ductility, malleability, high tensile strength thermal & electrical conductivity hard & brittle – partially filled d orbital covalent bonding between metal atom through orbital overlap zn, cd & hg – completely. Transition metals. mercury (hg) is the only transition metal that is not a solid. the transition metals all have valence electrons in a d subshell. like other metals, transition metals form cations not anions. we shall see that many transitions cations form beautifully coloured compounds. 646 views • 18 slides. Faculty. steven dessens. notes and practice problems. chem 1412 general chemistry ii (with lab) chem 1412 previous textbook powerpoints. chem 1412 chang powerpoints. chapter 22 transition metal chemistry and coordination compounds.

Ppt Transition Metals Powerpoint Presentation Free Downloadођ Transition metals. mercury (hg) is the only transition metal that is not a solid. the transition metals all have valence electrons in a d subshell. like other metals, transition metals form cations not anions. we shall see that many transitions cations form beautifully coloured compounds. 646 views • 18 slides. Faculty. steven dessens. notes and practice problems. chem 1412 general chemistry ii (with lab) chem 1412 previous textbook powerpoints. chem 1412 chang powerpoints. chapter 22 transition metal chemistry and coordination compounds. Transition metal ions are often coloured they. absorb em radiation because of loss of degeneracy. of d orbitals those which absorb in the visible. region will appear the complementary colour. 21. the 5 d orbitals in an isolated atom are. degenerate. ligands cause the d orbitals to become. non degenerate. Transition metal chemistry: crystal field theory jessica comstock kata haeberlin kelsey fisher transition metals elements in which the d and f subshells are – a free powerpoint ppt presentation (displayed as an html5 slide show) on powershow id: 3e9b58 mdk3y.
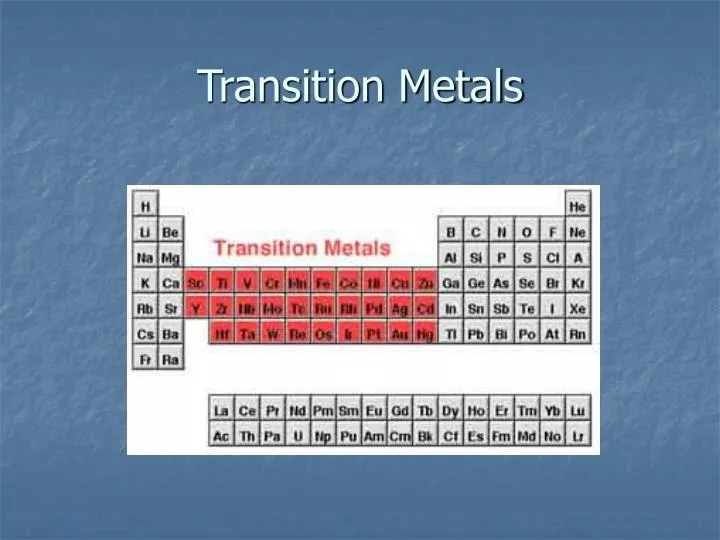
Ppt Transition Metals Powerpoint Presentation Free Downloadођ Transition metal ions are often coloured they. absorb em radiation because of loss of degeneracy. of d orbitals those which absorb in the visible. region will appear the complementary colour. 21. the 5 d orbitals in an isolated atom are. degenerate. ligands cause the d orbitals to become. non degenerate. Transition metal chemistry: crystal field theory jessica comstock kata haeberlin kelsey fisher transition metals elements in which the d and f subshells are – a free powerpoint ppt presentation (displayed as an html5 slide show) on powershow id: 3e9b58 mdk3y.
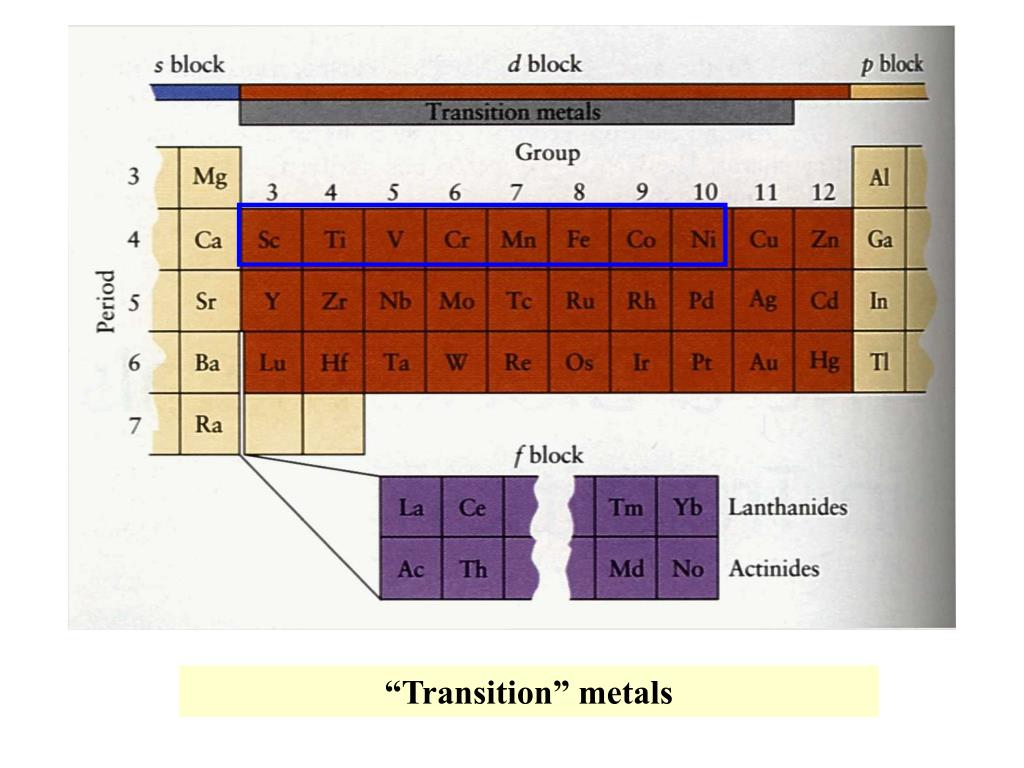
Ppt Chapter 20 The Transition Elements Powerpoint Presentation Free 21

Comments are closed.