Oxidation Reduction Reactions
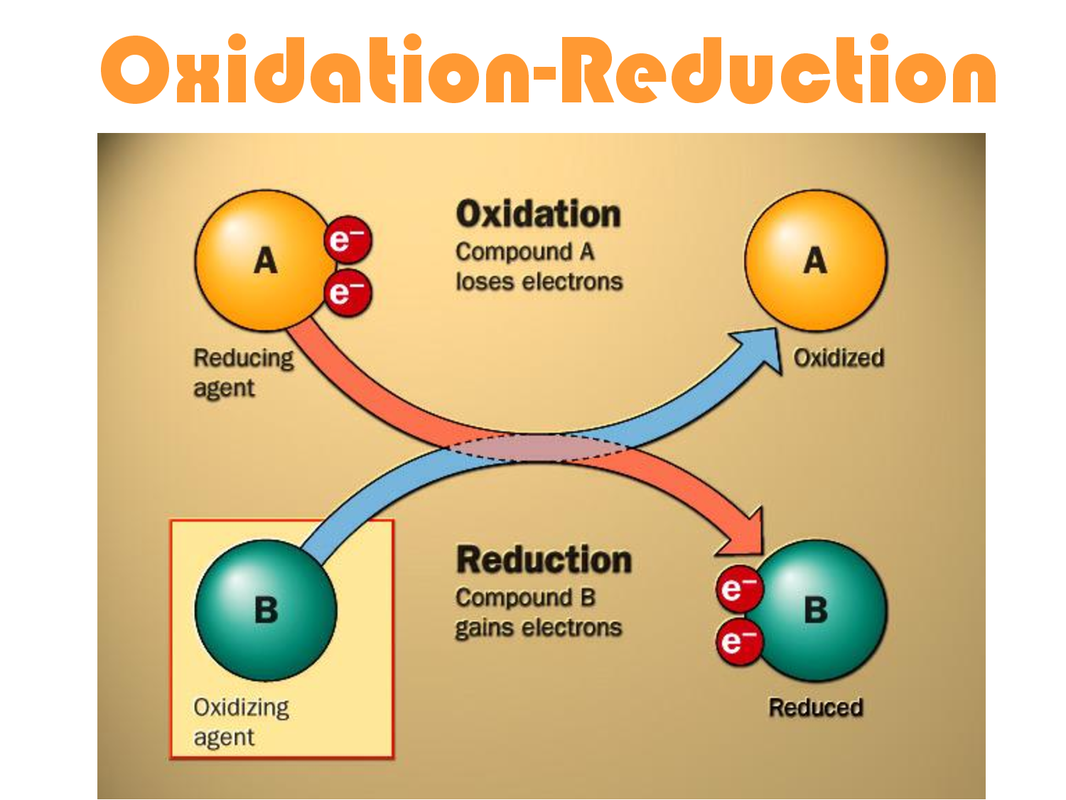
Titration Acid Base Oxidation Reduction At Tom Acuff Blog An oxidation reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. an oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. redox reactions are common and vital to some of the basic. Oxidation reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. many such reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration and photosynthesis—basic life functions.
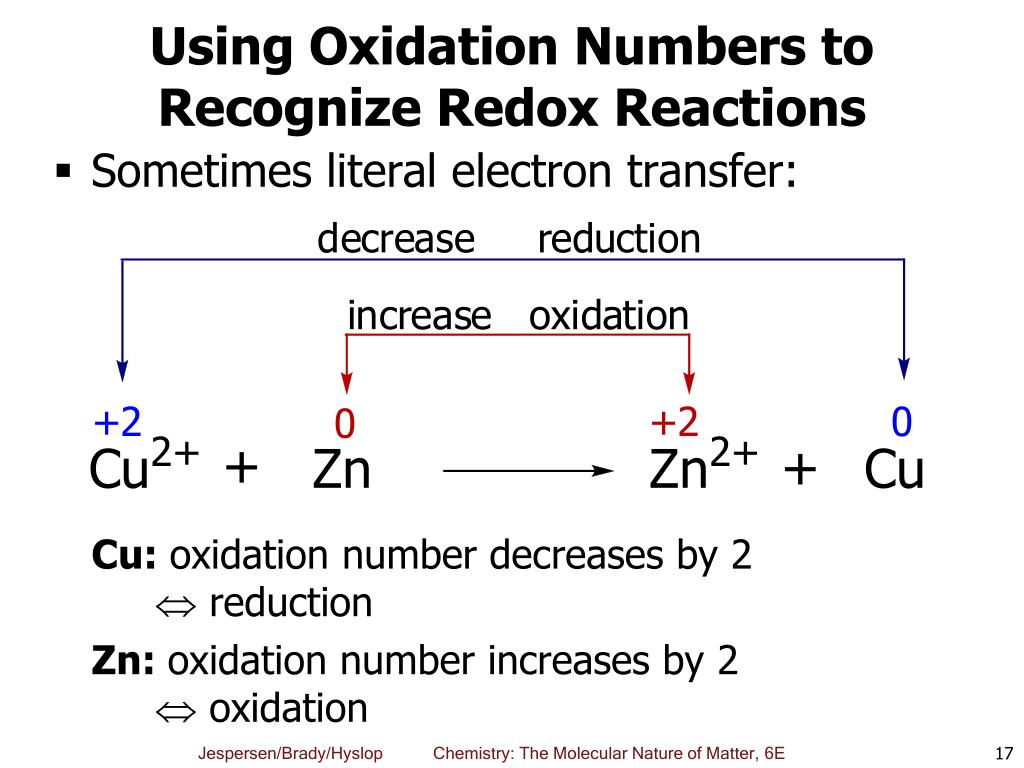
Ppt Chapter 6 Oxidation Reduction Reactions Powerpoint Presentation Redox ( ˈrɛdɒks red oks, ˈriːdɒks ree doks, reduction–oxidation[2] or oxidation–reduction[3]: 150 ) is a type of chemical reaction in which the oxidation states of the reactants change. [4] oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the. Learn the definition, examples, and types of redox reactions, where electrons are transferred between two species. find out the difference between oxidation and reduction, and how to write and recognize redox reactions. Write the balanced equation for the combustion of hexane, c 6 h 14. answer. 7.9: oxidation–reduction reactions ck 12. an oxidation reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another. oxidation is the full or partial loss of electrons or the gain of …. The reaction is as follows: cu2o(s) h2(g) → 2cu(s) h2o(g) (7.4.1) oxidation reduction reactions are now defined as reactions that exhibit a change in the oxidation states of one or more elements in the reactants by a transfer of electrons, which follows the mnemonic "oxidation is loss, reduction is gain", or "oil rig".
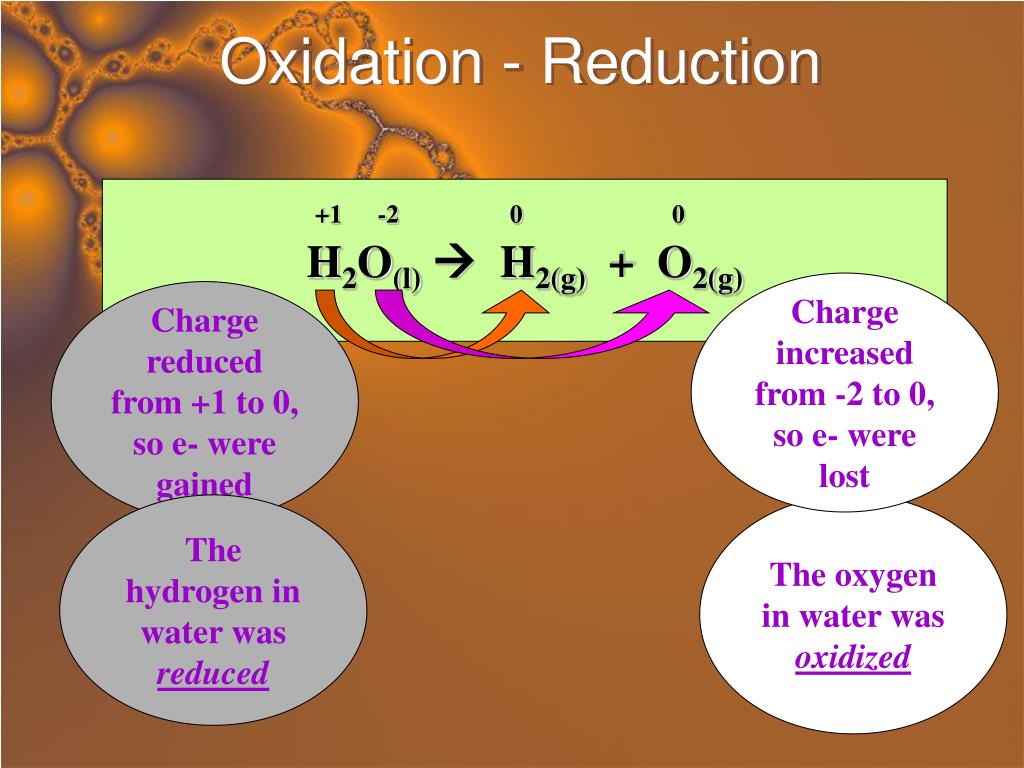
Ppt Chapter 19 Oxidation Reduction Reactions Powerpoint Write the balanced equation for the combustion of hexane, c 6 h 14. answer. 7.9: oxidation–reduction reactions ck 12. an oxidation reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another. oxidation is the full or partial loss of electrons or the gain of …. The reaction is as follows: cu2o(s) h2(g) → 2cu(s) h2o(g) (7.4.1) oxidation reduction reactions are now defined as reactions that exhibit a change in the oxidation states of one or more elements in the reactants by a transfer of electrons, which follows the mnemonic "oxidation is loss, reduction is gain", or "oil rig". Oxidation and reduction. oxidation involves an increase in oxidation number, while reduction involves a decrease in oxidation number. usually, the change in oxidation number is associated with a gain or loss of electrons, but there are some redox reactions (e.g., covalent bonding) that do not involve electron transfer. Learn the history, definitions and examples of oxidation reduction reactions, also known as redox reactions. find out how to assign oxidation numbers and recognize redox reactions in different contexts.

Comments are closed.