Oxidation Reduction Reaction Chemistry Libretexts
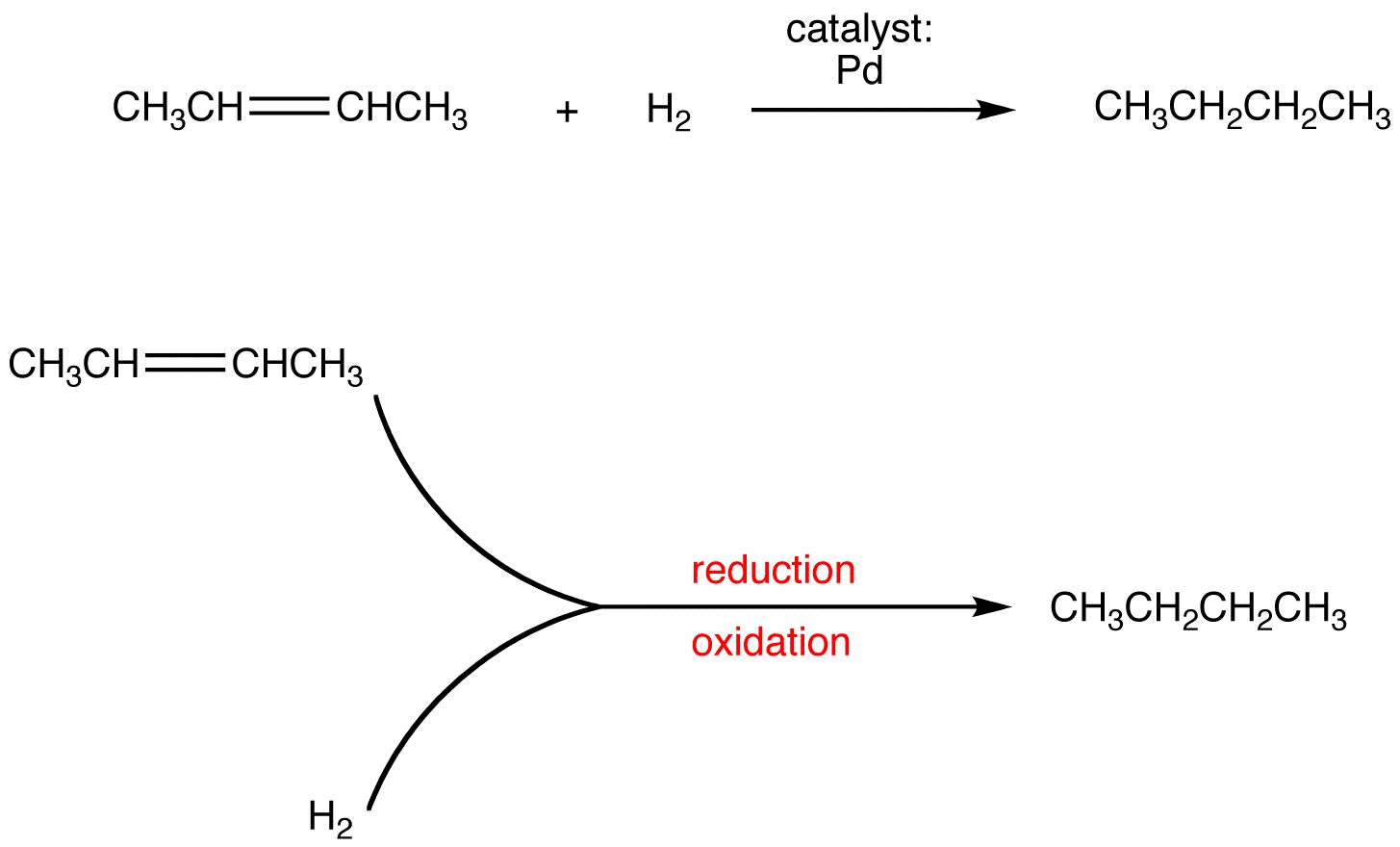
Oxidation Reduction Reaction Chemistry Libretexts An oxidation reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. an oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. redox reactions are common and vital to some of the basic. The corrosion process involves an oxidation–reduction reaction in which metallic iron is converted to fe (oh) 3, a reddish brown solid. many metals dissolve through reactions of this type, which have the general form. metal acid → salt hydrogen (4.4.8) (4.4.8) metal acid → salt hydrogen.
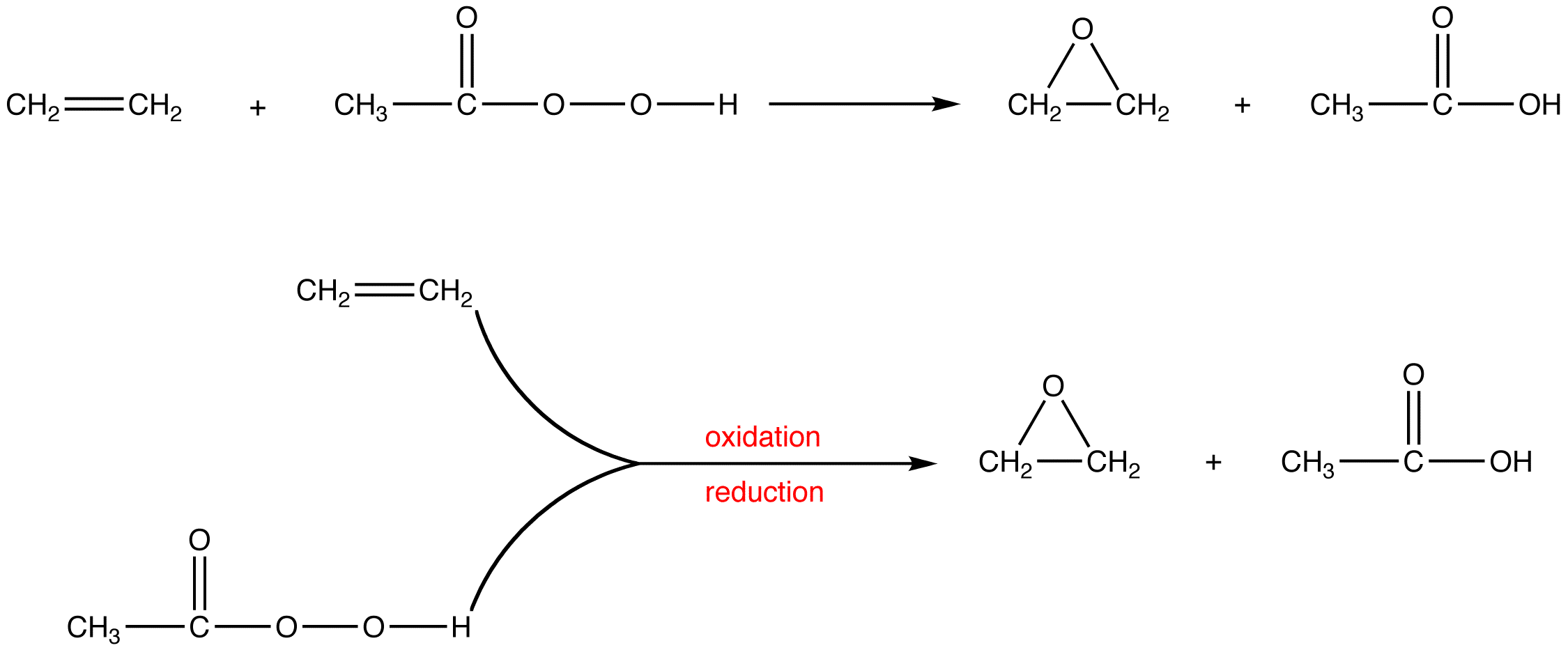
Oxidation Reduction Reaction Chemistry Libretexts To identify a chemical reaction as an oxidation reduction reaction. when zinc metal is submerged into a quantity of aqueous hcl hcl, the following reaction occurs (figure 6.5.1 6.5. 1): zn(s) 2hcl(aq) → h2(g) zncl2(aq) (6.5.1) (6.5.1) zn (s) 2 hcl (aq) → h 2 (g) zncl 2 (aq) this is one example of what is sometimes called a single. Ch4 2o2 → co2 2h2o (10.2.1) in respiration, the biochemical process by which the oxygen we inhale in air oxidizes foodstuffs to carbon dioxide and water, redox reactions provide energy to living cells. a typical respiratory reaction is the oxidation of glucose ( c6h12o6 ), the simple sugar we encountered in the chapter opening essay that. Redox reactions are identified per definition if one or more elements undergo a change in oxidation number. this is not a redox reaction, since oxidation numbers remain unchanged for all elements. this is a redox reaction. gallium is oxidized, its oxidation number increasing from 0 in ga (l) to 3 in gabr 3 (s). Oxidation reduction reactions are of central importance in organic chemistry and biochemistry. the burning of fuels that provides the energy to maintain our civilization and the metabolism of foods that furnish the energy that keeps us alive both involve redox reactions. figure \(\pageindex{1}\): the burning of natural gas.

Oxidation And Reduction Reactions Chemistry Libretexts Redox reactions are identified per definition if one or more elements undergo a change in oxidation number. this is not a redox reaction, since oxidation numbers remain unchanged for all elements. this is a redox reaction. gallium is oxidized, its oxidation number increasing from 0 in ga (l) to 3 in gabr 3 (s). Oxidation reduction reactions are of central importance in organic chemistry and biochemistry. the burning of fuels that provides the energy to maintain our civilization and the metabolism of foods that furnish the energy that keeps us alive both involve redox reactions. figure \(\pageindex{1}\): the burning of natural gas. Converting to cell potentials: − nfecell = − nfe ∘ cell rtlnq. or. ecell = e ∘ cell − rt nf lnq. equation 9.3.11 is the generalized nernst equation that is applicable at any temperature. however, is can be simplified for reactions occuring at 25 °c (298.15 k) by rewriting it as. ecell = e ∘ cell − 0.0257v n lnq. The libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. we also acknowledge previous national science foundation support.

Oxidationreduction Reaction Chemistry Libretexts Converting to cell potentials: − nfecell = − nfe ∘ cell rtlnq. or. ecell = e ∘ cell − rt nf lnq. equation 9.3.11 is the generalized nernst equation that is applicable at any temperature. however, is can be simplified for reactions occuring at 25 °c (298.15 k) by rewriting it as. ecell = e ∘ cell − 0.0257v n lnq. The libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. we also acknowledge previous national science foundation support.
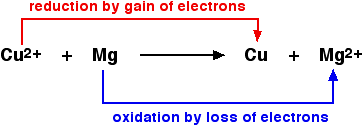
Definitions Of Oxidation And Reduction Chemistry Libretexts

Comments are closed.