Osmosis Definition Examples Facts Britannica

Osmosis Definition Examples Facts Britannica Osmosis, the spontaneous passage or diffusion of water or other solvents through a semipermeable membrane (one that blocks the passage of dissolved substances—i.e., solutes). the process, important in biology, was first thoroughly studied in 1877 by a german plant physiologist, wilhelm pfeffer. earlier workers had made less accurate studies. Human red blood cells placed in fresh water expand and burst. these are examples of the effects of osmosis, the process by which water passes through a cell membrane. osmosis is possible because of the constant state of motion that exists at the atomic and molecular levels of matter. specifically, in liquid solutions, molecules of solute (the.

Osmosis Definition Examples Facts Britannica вђ Bilarasa Homeostasis, any self regulating process by which biological systems tend to maintain stability while adjusting to conditions that are optimal for survival. if homeostasis is successful, life continues; if unsuccessful, disaster or death ensues. the stability attained is actually a dynamic equilibrium, in which continuous change occurs yet. An example of osmosis occurs when a sugar solution and water, top, are separated by a semipermeable membrane. the solution's large sugar molecules cannot pass through the membrane into the water. small water molecules move through the membrane until equilibrium is established, bottom. Osmosis happens spontaneously and without any energy on the part of the cell. solvents and solutes. osmosis deals with chemical solutions. solutions have two parts, a solvent and a solute. when solute dissolves in a solvent, the end product is called a solution. salt water is an example of a solution; salt is the solute, and water is the. Osmosis is the net movement of solvent molecules through a semipermeable membrane. it is similar to diffusion as the movement is downhill, meaning from higher to lower concentration. in osmosis though, the movement has to occur across a semipermeable or selectively permeable membrane. without this element, it cannot be called osmosis.
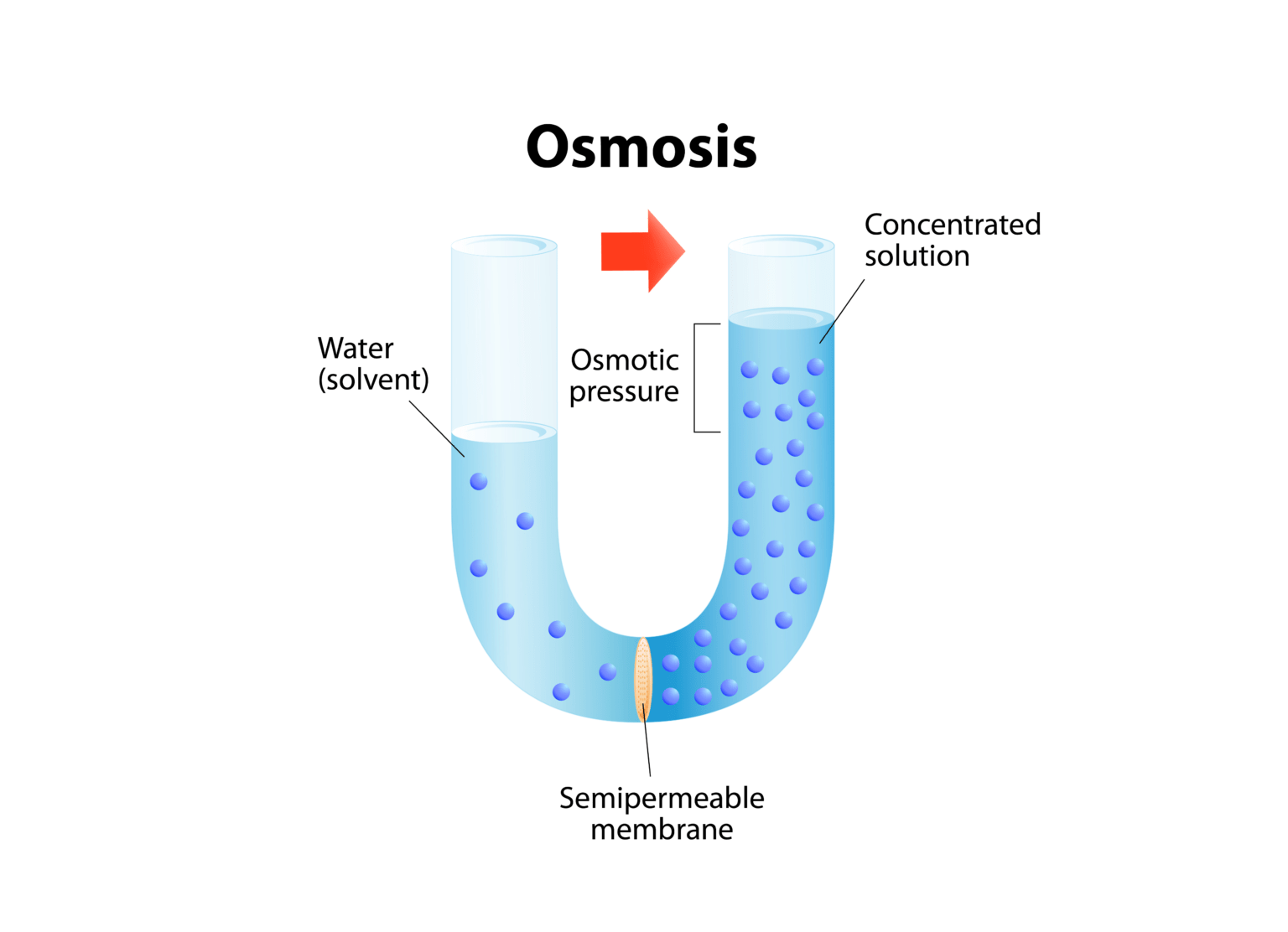
Osmosis Definition Examples Facts Britannica Vrogue Co Osmosis happens spontaneously and without any energy on the part of the cell. solvents and solutes. osmosis deals with chemical solutions. solutions have two parts, a solvent and a solute. when solute dissolves in a solvent, the end product is called a solution. salt water is an example of a solution; salt is the solute, and water is the. Osmosis is the net movement of solvent molecules through a semipermeable membrane. it is similar to diffusion as the movement is downhill, meaning from higher to lower concentration. in osmosis though, the movement has to occur across a semipermeable or selectively permeable membrane. without this element, it cannot be called osmosis. Basic characteristics of osmosis. requires a semipermeable membrane. a slow and spontaneous process. occurs in liquid medium. requires no energy expenditure and thus also called passive diffusion. movement of water occurs from a region of high water potential to a region of low water potential. the process continues until the concentration of. About the author. the process of osmosis is a type of diffusion that moves water molecules rather than solute across a semipermeable membrane, such as the cell membrane. osmotic pressure will equalize the amount of solute across a concentration gradient. hypertonic and hypotonic solutions affect cells differently.

Osmosis Definition Examples Facts Britannica Vrogue Co Basic characteristics of osmosis. requires a semipermeable membrane. a slow and spontaneous process. occurs in liquid medium. requires no energy expenditure and thus also called passive diffusion. movement of water occurs from a region of high water potential to a region of low water potential. the process continues until the concentration of. About the author. the process of osmosis is a type of diffusion that moves water molecules rather than solute across a semipermeable membrane, such as the cell membrane. osmotic pressure will equalize the amount of solute across a concentration gradient. hypertonic and hypotonic solutions affect cells differently.

Comments are closed.