Organic Chemistry Lab Report Number 1 Solvent Extraction And

Organic Chemistry Lab Report Number 1 Solvent Extraction And Organic chemistry lab report number 1 solvent extraction and partition coefficient part experiment 4a objective: the purpose of this lab is to get familiar with. Solvent extraction ryan huckaby chem 2123 2 27 dr. srinivasan 2123 2 27 solvent extraction abstract solvent extraction is a method used to separate and purify compounds. in the case of the this experiment the compound that will be extracted is dibenzyl acetone.

Solvent Extraction Lab Report Docx Organic Chemistry Lab I Extraction #1. perform a single extraction using approximately 25ml 25 ml of diethyl ether (an exact amount is not necessary), as described previously, making sure to appropriately label each layer (e.g. "top organic layer" and "bottom aqueous layer"). extraction #2. return the aqueous layer to the separatory funnel. Solvent extraction terpsichore maras lindeman ta matthew che 231 s. 001 february , 2011 s o l v e n t e x t r a c t i o n solvent extraction purpose a neutral compound, a base and an acid are separated using the process of solvent extraction. the three components of the our mixture once they have been extracted by the process of sol vent. The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. figure 4.16 shows a diagram of an aqueous solution being extracted twice with diethyl ether. diethyl ether has a density less than \(1 \: \text{g ml}\), so is the top organic layer in the funnel. 93158. lisa nichols. butte college. "extraction" refers to transference of compound (s) from a solid or liquid into a different solvent or phase. in the chemistry lab, it is most common to use liquid liquid extraction, a process that occurs in a separatory funnel. a solution containing dissolved components is placed in the funnel and an.
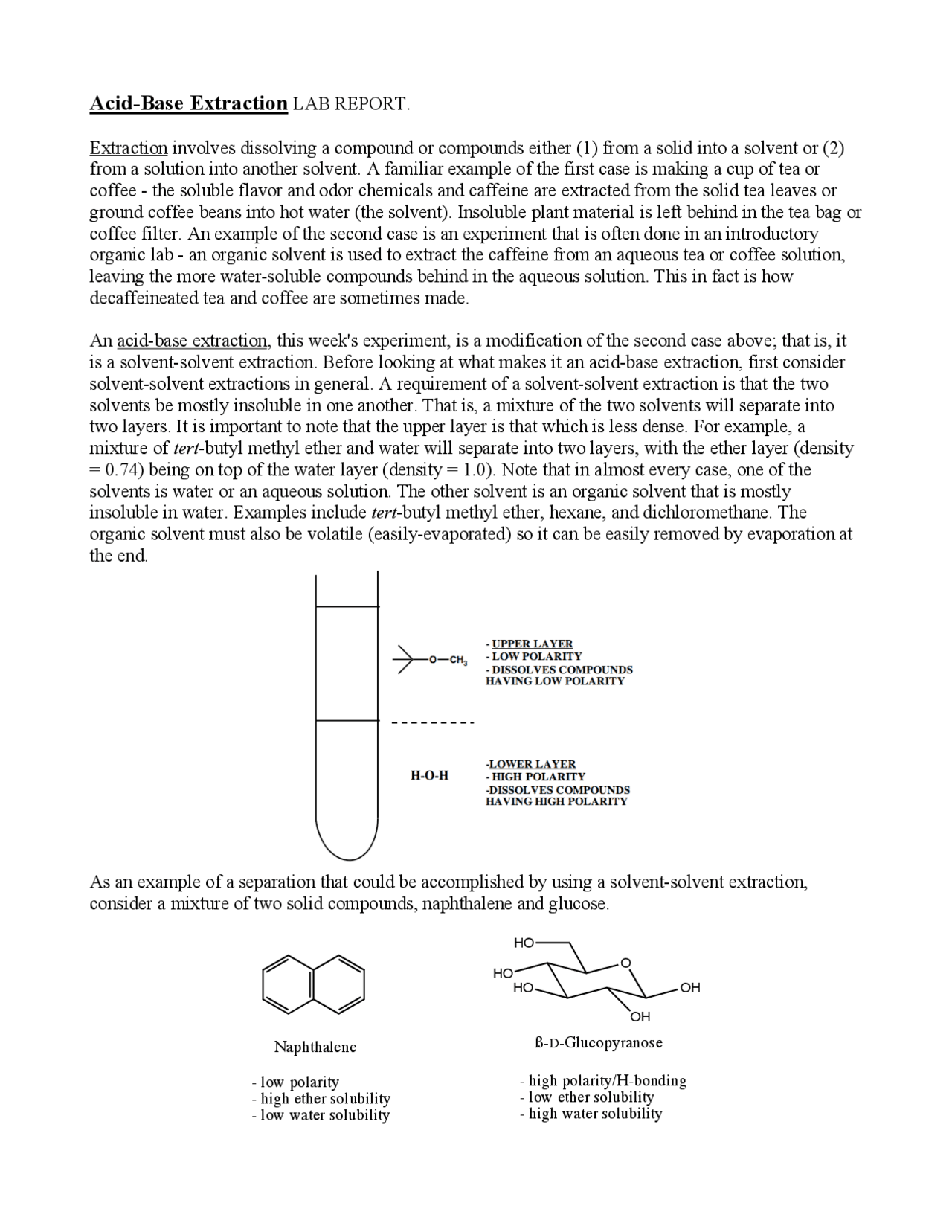
Acid Base Extraction Lab Report Organic Chemistry Docsity The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. figure 4.16 shows a diagram of an aqueous solution being extracted twice with diethyl ether. diethyl ether has a density less than \(1 \: \text{g ml}\), so is the top organic layer in the funnel. 93158. lisa nichols. butte college. "extraction" refers to transference of compound (s) from a solid or liquid into a different solvent or phase. in the chemistry lab, it is most common to use liquid liquid extraction, a process that occurs in a separatory funnel. a solution containing dissolved components is placed in the funnel and an. Experiment 8 – extraction pg. 1 6. extraction a. background extraction is a frequently used technique to selectively transfer a compound of interested from one solvent to another. extraction is based on solubility characteristics of the organic compound in the solvents being used for the extraction. let’s consider two frequently encountered. Rearranging this gives: x = 5 5x which solves as: x = 0.83 g in ether and 1 x = 0.17g in water if the extraction is carried out with the same amount of ether in two equal portions of 50 ml each then we have for the first extraction: 0 .71g in ether x 50. 5 ; (1 x ) 100 1 x 0 . 29g in water.

Comments are closed.