Organic Chemistry 255 Lab 3 Simple And Fractional Distillation Final

Organic Chemistry 255 Lab 3 Simple And Fractional Distillation Final This video follows the lab that would be performed in organic chemistry 1 at umb. this details the technique of distillation (fractional and simple) as well. Table 2: simple and fractional distillation for 10 % by volume of aqueous ethanol. simple distillation fractional distillation. 2 89 2 73. 6 98 6 98. 8 98 8 98. 23 99 23 99. 25 98 24 .0 99. 26 98 24 99. table 3: simple and fractional distillation for 30 % by volume of aqueous ethanol. simple distillation fractional distillation. 7 85 10 .0 78.
Illustrated Glossary Of Organic Chemistry Distillation Simple Lab #3: simple and fractional distillation. abstract. the aim of this experiment was to compare the effectiveness of simple distillation versus fractional distillation in separating a mixture containing two compounds with different boiling points by using the concept of theoretical plates. Table 1: simple and fractional distillation graph. 0 2 4 6 8 10 12 14 16 18 20 80. 85. 90. 95. 100. 105. 110. temperature vs volume of cyclohexane toluene mixture. volume of distillate (ml) temperture in c. a simple distillation shows a gradual increase of temperature with volume of distillate. A. a fractional distillation provides more theoretical plates than a simple distillation b. a simple distillation is suitable to separate two liquid compounds whose boiling points are 40 °c apart c. one purpose of a distillation is to separate a liquid from a dissolved solid. Distillation involves a series of vaportations and condinsations. what is distillation? a process that involves heating up and cooling a liquid. a process involving evaporation and condensation. in the condenser the cold water flows in from the top then comes out the bottom. (true or false) false. what is the technique we use in distillation.

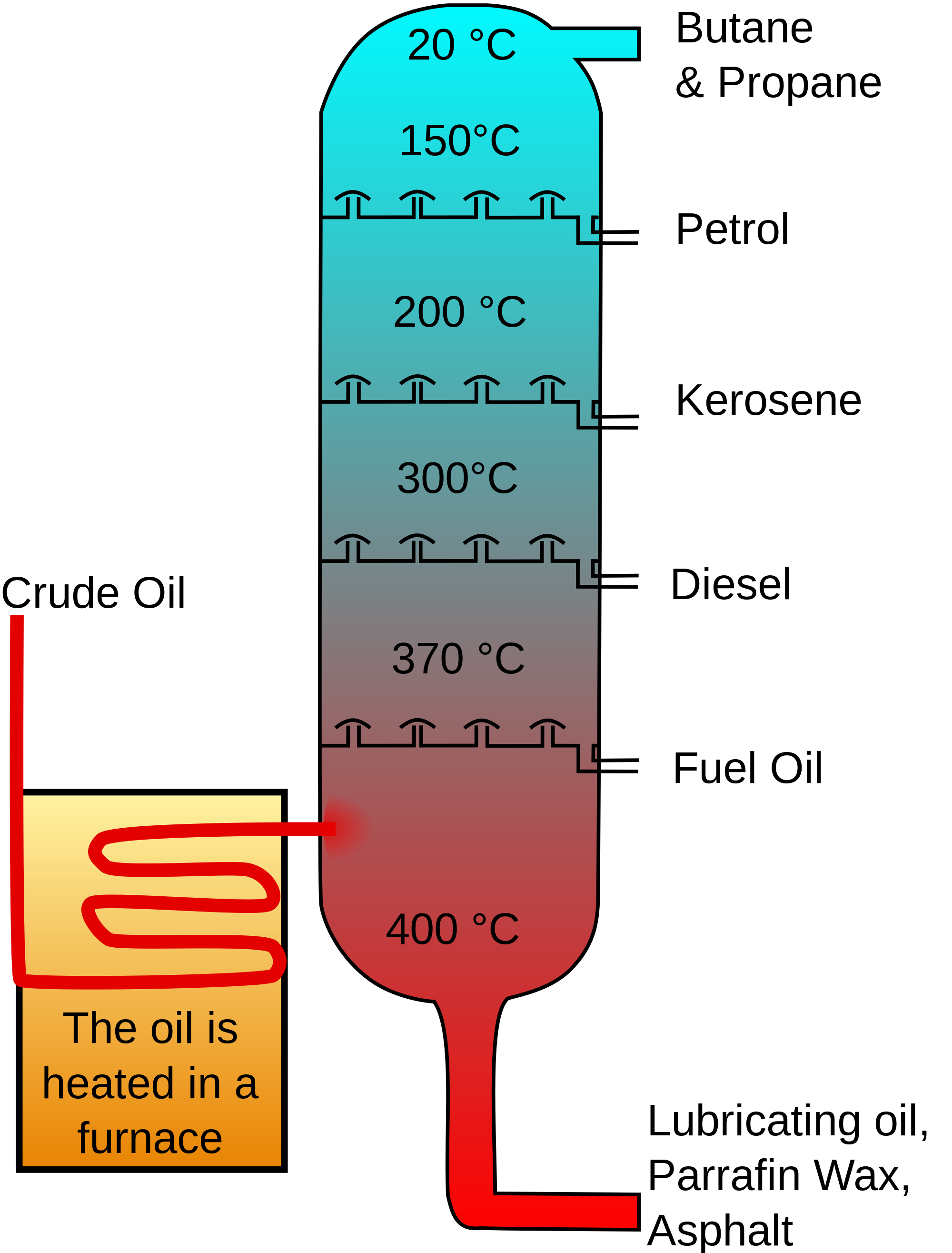
What Is The Distillation Process The Chemistry Blog A. a fractional distillation provides more theoretical plates than a simple distillation b. a simple distillation is suitable to separate two liquid compounds whose boiling points are 40 °c apart c. one purpose of a distillation is to separate a liquid from a dissolved solid. Distillation involves a series of vaportations and condinsations. what is distillation? a process that involves heating up and cooling a liquid. a process involving evaporation and condensation. in the condenser the cold water flows in from the top then comes out the bottom. (true or false) false. what is the technique we use in distillation. 1. stock mixture of solvents needs to be boiled gradually, simple distillate. 2. simple distillation is used to separate solvents with a large boiling point difference. 3. a condenser must be used between the distillation head and the adaptor. 4. simple distillation can be used to separate a volatile compound from nonvolatile impurities. 5.3c: uses of fractional distillation. fractional distillation is used for both oil refining and purification of reagents and products. fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties.

Simple And Fractional Distillation Ks3 Chemistry Revision 1. stock mixture of solvents needs to be boiled gradually, simple distillate. 2. simple distillation is used to separate solvents with a large boiling point difference. 3. a condenser must be used between the distillation head and the adaptor. 4. simple distillation can be used to separate a volatile compound from nonvolatile impurities. 5.3c: uses of fractional distillation. fractional distillation is used for both oil refining and purification of reagents and products. fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties.

Comments are closed.