Orbital Overlap Diagram Of Co2 Carbon Dioxide
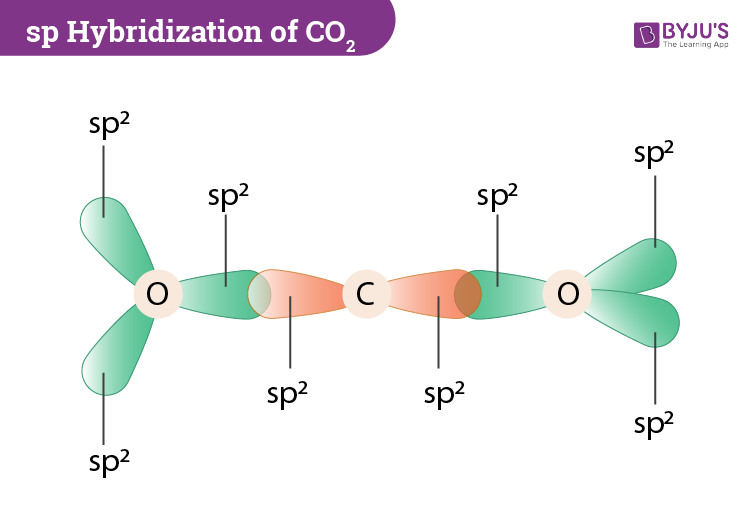
Co2 Orbital Hybridization The carbon atom is double bonded to each of the two oxygens.you'll need to show an unhybridized 2p orbital in an up down direction (i label it 2py here) and. The mo diagram for carbon dioxide shows that the carbon oxygen double bonds are formed by the overlap of the carbon atom’s 2p orbital with the oxygen atom’s 2p orbitals. these bonds result in the sharing of electron density between the carbon and oxygen atoms, creating the stable structure of carbon dioxide.

How To Draw Molecular Orbital Diagram Of Co2 Study With knowledge of both orbital symmetries and energies, we can construct the molecular orbital diagram. the carbon atom goes on one side of the diagram while the oxygen salcs are drawn on the opposite side. molecular orbitals are drawn in the center column of the diagram: figure \(\pageindex{3}\): molecular orbital diagram for carbon dioxide. The molecular orbital (mo) diagram of carbon dioxide (co2) represents the energy levels and electron occupancy in the molecule. it helps in understanding the bonding and electronic structure of co2. in the mo diagram of co2, there are a total of six molecular orbitals formed from the overlapping of the atomic orbitals of carbon and oxygen atoms. In the case of co2, the molecular orbital diagram shows that there are two electrons in the σ bonding orbitals and four electrons in the π bonding orbitals. thus, the bond order of co2 is (2 – 4) 2 = 1. this negative bond order indicates that the molecule has an unstable, antibonding interaction. as a result, co2 is a highly reactive molecule. Molecular orbital (mo) theory is used by scientists to understand bonding in molecules. carbon dioxide is a linear, centrosymmetric molecule with d ∞h symmetry. surrounded by two oxygens, carbon is the central atom. mo therory predicts π bond formation resulting from the interaction of c 2p x and y atomic orbitals with o fragment lgo (ligand.

Orbital Overlap Diagram Of Co2 Carbon Dioxide Youtube In the case of co2, the molecular orbital diagram shows that there are two electrons in the σ bonding orbitals and four electrons in the π bonding orbitals. thus, the bond order of co2 is (2 – 4) 2 = 1. this negative bond order indicates that the molecule has an unstable, antibonding interaction. as a result, co2 is a highly reactive molecule. Molecular orbital (mo) theory is used by scientists to understand bonding in molecules. carbon dioxide is a linear, centrosymmetric molecule with d ∞h symmetry. surrounded by two oxygens, carbon is the central atom. mo therory predicts π bond formation resulting from the interaction of c 2p x and y atomic orbitals with o fragment lgo (ligand. What is the orbital diagram of co2? the orbital diagram of co2 is a visual representation of the arrangement of electrons in the molecular orbitals of carbon dioxide. it helps to understand the bonding and structure of co2 at the atomic level. in the orbital diagram, the molecular orbitals are represented by boxes or lines, and the electrons by. The isosurface viewer below illustrates the valence bond theory description of bonding in the carbon dioxide molecule. the small red spheres on the right and left indicate the positions of the oxygen nuclei. the gray sphere marks the position of the carbon nucleus. examine each of the atomic orbitals. identify orbitals for lone pairs of electrons.

9 6 The Hybrid Orbital Model Chemwiki What is the orbital diagram of co2? the orbital diagram of co2 is a visual representation of the arrangement of electrons in the molecular orbitals of carbon dioxide. it helps to understand the bonding and structure of co2 at the atomic level. in the orbital diagram, the molecular orbitals are represented by boxes or lines, and the electrons by. The isosurface viewer below illustrates the valence bond theory description of bonding in the carbon dioxide molecule. the small red spheres on the right and left indicate the positions of the oxygen nuclei. the gray sphere marks the position of the carbon nucleus. examine each of the atomic orbitals. identify orbitals for lone pairs of electrons.
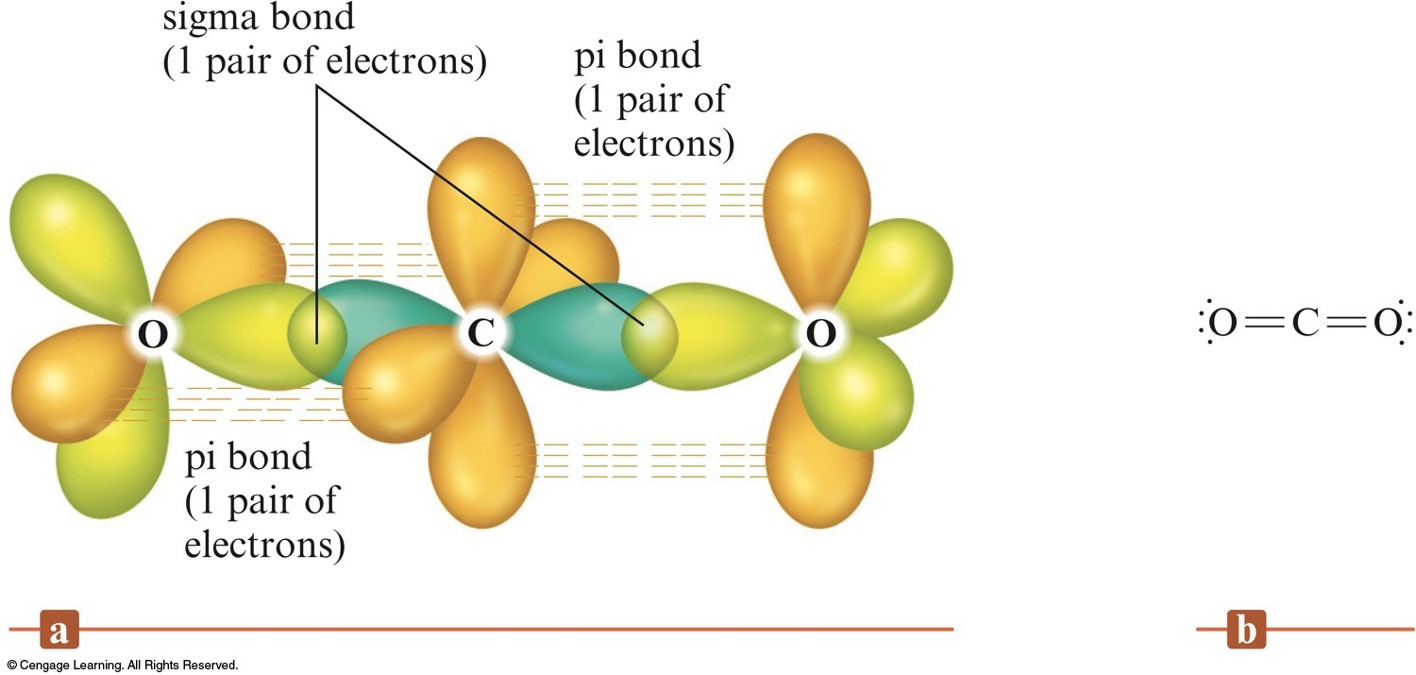
Chapter 9 Presentation

Comments are closed.