O1 Extraction Of Organic Compounds Youtube

O1 Extraction Of Organic Compounds Youtube O1 extraction of organic compounds=====وحدة التعليم الإلكتروني كلية الزراعة – جامعة المنصورة=====ضمن. When we perform a chemical reaction, we are usually trying to get a particular molecule. but when we are done with the reaction, there will probably be a bun.

Extraction Of Organic Compound Experiment Illustration Stock Photo Alamy If you find this video helpful, you might want to visit the uco chemistry channel, where you can find other video playlists for organic lab videos, most of t. Add the extracting solvent and stopper the funnel. two layers should now be in evidence. be sure you know which layer is which. ordinarily, one layer is aqueous (polar) and one is organic (non polar), with the organic layer being less dense and thus the top layer. remove the funnel from the stand, and hold it horizontally with a gentle but firm. The calculation for the third extraction is as follows: 4.07 = (x 50ml ether) (0.09g − x 150 ml water) after solving the algebra, x = 0.05g. this result means 0.04g remains in the aqueous layer (0.09g − 0.05g) after the third extraction. the results of the calculations in this section are summarized in figure 4.18. Extraction #1. perform a single extraction using approximately 25ml 25 ml of diethyl ether (an exact amount is not necessary), as described previously, making sure to appropriately label each layer (e.g. "top organic layer" and "bottom aqueous layer"). extraction #2. return the aqueous layer to the separatory funnel.
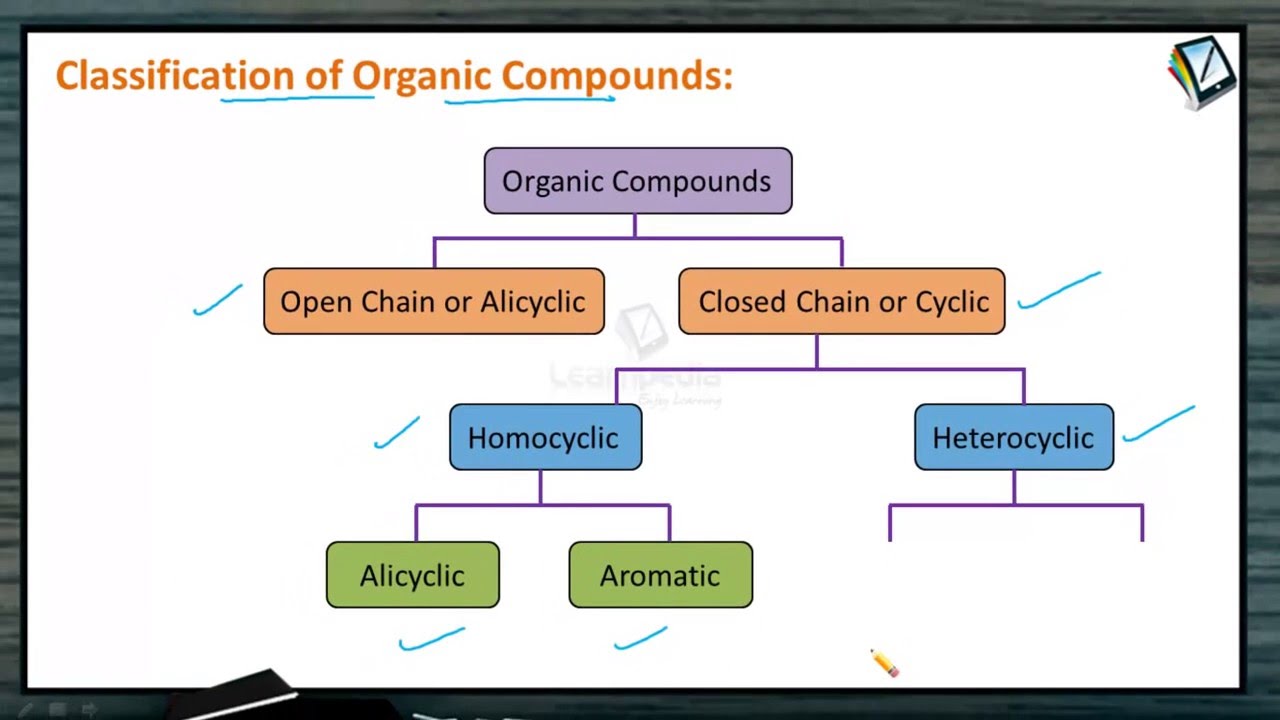
Classification Of Organic Compounds Youtube The calculation for the third extraction is as follows: 4.07 = (x 50ml ether) (0.09g − x 150 ml water) after solving the algebra, x = 0.05g. this result means 0.04g remains in the aqueous layer (0.09g − 0.05g) after the third extraction. the results of the calculations in this section are summarized in figure 4.18. Extraction #1. perform a single extraction using approximately 25ml 25 ml of diethyl ether (an exact amount is not necessary), as described previously, making sure to appropriately label each layer (e.g. "top organic layer" and "bottom aqueous layer"). extraction #2. return the aqueous layer to the separatory funnel. A commonly used method of separating a mixture of organic compounds is known as liquid liquid extraction. most reactions of organic compounds require extraction at some stage of product purification. in this experiment you will use extraction techniques to separate a mixture of an organic acid, a base, and a neutral compound. organic acids and bases can be separated from each other and from. 93158. lisa nichols. butte college. "extraction" refers to transference of compound (s) from a solid or liquid into a different solvent or phase. in the chemistry lab, it is most common to use liquid liquid extraction, a process that occurs in a separatory funnel. a solution containing dissolved components is placed in the funnel and an.

Extraction Of Compounds A commonly used method of separating a mixture of organic compounds is known as liquid liquid extraction. most reactions of organic compounds require extraction at some stage of product purification. in this experiment you will use extraction techniques to separate a mixture of an organic acid, a base, and a neutral compound. organic acids and bases can be separated from each other and from. 93158. lisa nichols. butte college. "extraction" refers to transference of compound (s) from a solid or liquid into a different solvent or phase. in the chemistry lab, it is most common to use liquid liquid extraction, a process that occurs in a separatory funnel. a solution containing dissolved components is placed in the funnel and an.

Separating Components Of A Mixture By Extraction Youtube

Comments are closed.