New Blood Test Can Help Test For Colon Cancer
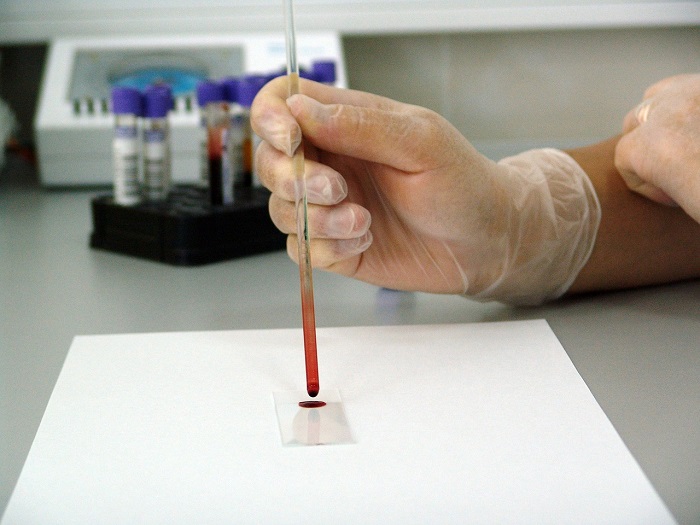
Breakthrough In New Blood Test For Colon Cancer On july 29, 2024, the food and drug administration (fda) approved a blood test that can help with primary colorectal cancer screening. the test is guardant health’s shield blood test, and the. July 29, 2024, 4:49 am pdt. by erika edwards. the food and drug administration on monday approved guardant health’s blood test, called shield, to screen for colon cancer. the test isn't meant to.

Colorectal Cancer New Blood Test May Help Determine If Chemother The blood test can detect dna shed into the bloodstream from tumors, and in a trial of more than 7,800 people, the new test accurately detected colon cancer at early, treatable stages 87% of the time. The fda approved the first blood test for screening colorectal cancer. in a clinical trial, the test identified 83% of the colorectal cancer cases and delivered false positive results to 10% of cancer free participants. the blood test isn't a substitute for a colonoscopy, but it could improve screening rates among people who are resistant to. The fda has approved a new colorectal cancer blood test by guardant health for medicare coverage. the test, called shield, looks for “free floating” fragments of cancer dna in the bloodstream. A new screening test can detect colorectal cancer with a simple blood draw in people with an average risk of the cancer. the test can detect early stage colorectal cancer with a sensitivity of 93%, according to mid stage data shared by the test maker, universal dx, in may. the test can also detect the pre cancerous growths with 54% sensitivity.

Comments are closed.