Ncl3 Lewis Structure Formal Charges
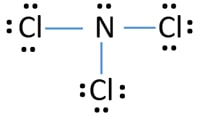
Ncl3 Nitrogen Trichloride Lewis Structure Formal charge = valence electrons – unbonded electrons – ½ bonded electrons. for, nitrogen = 5 – 2 – 1 26 = 0. chlorine = 7 6 1 22 = 0. from this, it is clear that the overall formal charge distribution on nitrogen trichloride is zero by which it should have been a nonpolar molecule. Example \(\pageindex{1}\): calculating formal charge from lewis structures. assign formal charges to each atom in the interhalogen ion \(\ce{icl4 }\). s olution. we divide the bonding electron pairs equally for all \(\ce{i–cl}\) bonds: we assign lone pairs of electrons to their atoms. each cl atom now has seven electrons assigned to it, and.
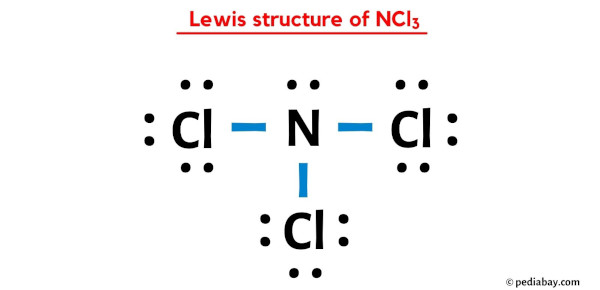
Ncl3 Lewis Structure Formal Charges Here’s how you can easily draw the ncl 3 lewis structure step by step: #1 draw a rough skeleton structure. #2 mention lone pairs on the atoms. #3 if needed, mention formal charges on the atoms. now, let’s take a closer look at each step mentioned above. A step by step description on how to calculate formal charges. formal charges are important because they allow us to predict which lewis structure is the mo. Step #1: calculate the total number of valence electrons. here, the given molecule is ncl3 (nitrogen trichloride). in order to draw the lewis structure of ncl3, first of all you have to find the total number of valence electrons present in the ncl3 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). Resonance forms. resonance hybrid. this page titled 4.4: formal charges and resonance is shared under a cc by license and was authored, remixed, and or curated by openstax. in a lewis structure, formal charges can be assigned to each atom by treating each bond as if one half of the electrons are assigned to each atom.

Lewis Structure Of Ncl3 Nitrogen Trichloride Youtube Step #1: calculate the total number of valence electrons. here, the given molecule is ncl3 (nitrogen trichloride). in order to draw the lewis structure of ncl3, first of all you have to find the total number of valence electrons present in the ncl3 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). Resonance forms. resonance hybrid. this page titled 4.4: formal charges and resonance is shared under a cc by license and was authored, remixed, and or curated by openstax. in a lewis structure, formal charges can be assigned to each atom by treating each bond as if one half of the electrons are assigned to each atom. Step 1: sketch the molecule – nitrogen trichloride with a center nitrogen atom surrounded by three chlorine atoms (3 n cl bonds) in a trigonal pyramidal arrangement. to draw the lewis structure of nitrogen trichloride ncl3, we first need to determine the number of valence electrons in each atom. nitrogen (n) is in group 5a of the periodic. Nitrogen trichloride (ncl3) lewis structure contains three n cl bonds. there is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. lewis structure of ncl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. also, there are no charges on atoms in ncl3. steps of drawing the lewis structure of ncl3 are.

Comments are closed.